The Personal Website of Mark W. Dawson

Containing
His Articles, Observations, Thoughts, Meanderings,
and some would say Wisdom (and some would say not).
Atomic Physics
As seen in the
Biographical History of the greatest Scientists and
Mathematicians
involved in the development of Atomic Physics
This outline utilizes no mathematics and only provides cursory information on The science of Atomic Physics.
- Introduction
- The Ages of Physics
- Background
- Galileo Galilei
- Isaac Newton
- Christiaan Huygens
- Michael Faraday
- James Clerk Maxwell
- Ludwig Boltzmann
- Max Planck
- Albert Einstein
- Neils Bohr
- Max Born
- Werner Heisenberg
- Erwin Schrodinger
- Paul Dirac
- Modern Quantum Theory
- Additional Thoughts
- Final Thoughts
- Further Readings
- Particle Physics Timeline
- The Noble Prize in Physics
- Disclaimer
Introduction
This article is an overview of the development of Atomic Physics. It is done by focusing on a the scientists who were crucial in its development. Although thousands of scientists have contributed to the development of Atomic Physics the thirteen scientists presented here have made such a significant impact as to change the course of Atomic Physics. It is, therefore, a biographical history of atomic physics. The scientific information is presented in general terms, and there is no mathematics. For those wishing more scientific details and/or mathematics, I have included a Further Readings section of books I would recommend that have more details and are readable by the general public.
I should point out that I am NOT a scientist, nor have I received any education or training in the sciences. This paper is the result of my readings on this subject in the past decades. Many academics and scientist would critique what I have written here as not accurate nor through. I freely acknowledge that these critiques are correct. It was not my intentions to be accurate or through, as I am not qualified to give an accurate nor through description. My intention was to be understandable to a layperson so that they can grasp the concepts. Academics and scientist entire education and training are based on accuracy and thoroughness, and as such, they strive for this accuracy and thoroughness. I believe it is essential for all laypersons to grasp the concepts of Atomic Physics, so they make more informed decisions on those areas of human endeavors that deal with science, engineering, and technology. As such, I did not strive for accuracy and thoroughness, only understandability.
The Ages of Physics
Science in human history can be broken down into three ages; Mythological, Aristotelian, and Galilean. Each age represents a different way to explain how and why the universe works the way it does.
Mythological Age
Before any formalization of science, people explained the ways of the universe through stories we refer to as myths. Their explanations were usually in the form of the Gods being displeased and causing bad things to happen, the Gods being please and causing good things to happen, and the Gods being indifferent and normal things happened. Although minor science was done (the Egyptians and the Babylonians come to mind) there was no systematic approach to determine why and how the universe worked.
Aristotelian Age
The Ancient Greeks were the first people to develop a systematic approach to the question as to why and how the universe worked. They utilized a philosophical approach as they thought about the question and developed logical reasoning to derive an answer. So, if their answer made logical sense it must be correct. They had no inclination to challenge their answer through observation or experimentation. The pinnacle of this approach was with the Philosopher Aristotle (for whom the age is named). Although some Ancient Greek philosophers did minor observations and experiments this was not the acceptable means of proving your answers.
Greek philosophers Leucippus and Democritus first developed the concept of the atom in the 5th century B.C.E. However, since Aristotle and other prominent thinkers of the time strongly opposed their idea of the atom their theory was overlooked and essentially buried until the 16th and 17th centuries. The Ancient Greek ideas on the atom were mostly all wrong, and not much thought was given to Atomic Physics until the Galilean Age. It is for this reason that this paper begins its discussion at the Galilean Age.
If you are interested in the Ancient Greek ideas on the atom I would encourage you to visit the website The Greek Concept of Atomos: The Indivisible Atom, which provides a good general public explanation of Ancient Greek thought on Atoms.
Galilean Age
Galileo Galilei was the first truly modern scientist, as he approached science by observing nature, performing experiments, developing a hypothesis utilizing mathematics, and testing his hypothesis to determine if it was correct. He was so good at this, and so correct, that other scientists adopted his methods as the way to do science. So much so that scientist who utilized these methods were known as "Natural Philosophers", and the Ancient Greek methods practitioners became known as simply "Philosophers".
Background
The Four Forces of Nature
The following are the four basic forces in nature. They were created at the time of the Big Bang and separated from themselves very shortly after they were created. This paper is about the first three forces (Atomic Physics), with Gravity being a focus in another paper.
- Electro-Magnetic Force - That acts between electrically charged particles. Electricity, magnetism, and light (the Electromagnetic Spectrum) are all produced by this force, and it has infinite range.
- Strong Force - That force that binds neutrons and protons together in the cores of atoms and is a very short-range force.
- Weak Force - That force that causes Beta decay, the release of atomic particles from an atom. Various atomic particles are formed by strong interactions but decay via the weak force. Like the strong force, the weak force is also a very short-range force.
- Gravity - That force that acts between all masses in the universe and it has infinite range. Gravity exists everywhere but is by many magnitudes weaker than all the other forces.
An Important Factor in the Advancement of Science
One of the most important factors in advancing science is the development of instruments to measure scientific phenomenon. Instruments that have a greater precision of length and/or a greater precision of time. Some early examples are the Refracting Telescope as refined by Galileo Galilei, the Reflecting Telescope as invented by Isaac Newton, the Pendulum Clock as invented by Christiaan Huygens, and the Microscope as invented by Zacharias Janssen and Hans Lippershey. Advancements in instrumentation led to advancements in science. This paper will not focus on these instrumentation advancements, rather the advancements in science that occurred as a result.
Galileo Galilei
Galileo studied speed and velocity, gravity and free fall, the principle of relativity, inertia, projectile motion and also worked in applied science and technology, describing the properties of pendulums and "hydrostatic balances", inventing the thermoscope and various military compasses, and using the telescope for scientific observations of celestial objects. His contributions to observational astronomy include the telescopic confirmation of the phases of Venus, the discovery of the four largest satellites of Jupiter, the observation of Saturn's rings (though he could not see them well enough to discern their true nature) and the analysis of sunspots. Known for his work as astronomer, physicist, engineer, philosopher, and mathematician, Galileo has been called the "Father of Observational Astronomy", the "Father of Modern Physics", the "Father of the Scientific Method", and even the "Father of Science".
|
Galileo Galilei was the first major scientist that challenged the Aristotelian method of science. He believed that "Observation and Experimentation" should be the basis of all science, and that the science was more important than philosophy or theology. If philosophy or theology did not agree with the science, then the philosophy or theology needed to be modified or discarded.
He challenged all Aristotelian science and proved with observation and experimentation that Aristotle was incorrect. He also answered many unanswerable problems (within his time) in science through observation and experimentation. He developed a scientific method that was the basis for all future scientific investigation, and he applied mathematics to science.
His defense of the Copernican revolution shook the chokehold of religion over science and allowed many other scientists to defend the Copernican revolution.
"The Dialogue Concerning the Two Chief World Systems", published in 1632, is the book by Galileo Galilei comparing the Copernican system with the traditional Ptolemaic system, and it was a significant factor in establishing the Copernican system.
"The Discourses and Mathematical Demonstrations Relating to Two New Sciences", published in 1638, was Galileo's final book and a scientific testament covering much of his work in physics over the preceding thirty years. It was much referred to by the succeeding generation of physicists.
His contribution to Atomic Physics was minimal, but without his discarding of Aristotelian science and loosening the chokehold of religion over science it would not have been possible to proceed with Atomic Physics.
Galileo's Character
Galileo seems to have led a fairly normal lifestyle for his time, and he had many friends, as well as contacts with other Natural Philosophers. However, when Galileo believed himself to be right he could become stubborn, persistent and argumentative in expressing his opinions. This often led him into conflicts with authority, and in his time authority was mainly the Catholic Church and the Princes of the principalities of Italy. He believed that if science conflicted with church doctrine, then church doctrine need to be changed or replaced. Unfortunately, Galileo lived during the time of the Protestant Reformation1, and the Catholic Church was not receptive to changing its doctrine. Galileo persisted and eventually incurred the ire of the Pope and he was forced to face the Inquisition2 in 1633. At the conclusion of the Inquisition, he was forced to recant his opinion and was placed under house arrest for the rest of his life. While under house arrest he completed his book "Two New Sciences", in which he summarized work he had done some forty years earlier on the two sciences now called kinematics and strength of materials.
As to his personal life, Galileo had a mistress, Marina Gamba, with whom he had three children. During one of his frequent trips to Venice, Galileo met a young woman named Marina di Andrea Gamba and started a relationship with her. She moved into his house in Padua and bore him three children: Virginia (16 August 1600 - 1634), later Sister Maria Celeste; Livia (1601 - 1659), later Sister Arcangela; and Vincenzo (1606 - 1649). In none of the three baptismal records is Galileo named as the father. Virginia was described as "daughter by fornication of Marina of Venice," with no mention of the father; on Livia's baptismal record the name of the father was left blank; Vincenzo's baptismal record announced "father uncertain". Galileo's position as a professor and his many friendships among the Venetian nobility probably made it unwise for him to figure officially as the children's father.
When Galileo left Padua for good in 1610 to take up his position at the Medici court in Florence, he took the two daughters with him but left their mother behind with 4 year-old Vincenzo, who joined his father in Florence a few years later.
With Marina no longer in the family, Galileo put his two daughters in a convent and managed to have Vincenzo legitimated by the Grand Duke of Tuscany. In his 1619 request for this, Galileo declared that at the time of his cohabitation with Marina, she "had never been married" and was "already dead" at the drawing up of the act.
Maria Celeste maintained contact with her father throughout her entire life through letters. Although none of Galileo's letters are known to have survived, 120 of Maria Celeste's exist. These letters, written from 1623 to 1634, depict a woman with incredible brilliance, industry, sensibility and a deep love for her father. Maria Celeste died of dysentery in 1634.* * * * *
1The Reformation, specifically referred to as the Protestant Reformation, was a schism in Western Christianity initiated by Martin Luther and continued by John Calvin, Huldrych Zwingli, and other early Protestant Reformers in 16th-century Europe. It is usually considered to have started with the publication of the Ninety-five Theses by Martin Luther in 1517 and lasted until the end of the Thirty Years' War with the Peace of Westphalia in 1648.
2The Inquisition was a group of institutions within the government system of the Catholic Church whose aim was to combat heresy. It started in 12th-century France to combat religious dissent, in particular the Cathars and the Waldensians. Other groups investigated later included the Spiritual Franciscans, the Hussites (followers of Jan Hus) and the Beguines. Beginning in the 1250s, inquisitors were generally chosen from members of the Dominican Order, replacing the earlier practice of using local clergy as judges. The term Medieval Inquisition covers these courts up to mid-15th century.
During the Late Middle Ages and early Renaissance, the concept and scope of the Inquisition significantly expanded in response to the Protestant Reformation and the Catholic Counter-Reformation. It expanded to other European countries, resulting in the Spanish Inquisition and Portuguese Inquisition. The Spanish and Portuguese operated inquisitorial courts throughout their empires in Africa, Asia, and the Americas (resulting in the Peruvian Inquisition and Mexican Inquisition). The Spanish and Portuguese inquisitions focused particularly on the issue of Jewish anusim and Muslim converts to Catholicism, partly because these minority groups were more numerous in Spain and Portugal than in many other parts of Europe, and partly because they were often considered suspect due to the assumption that they had secretly reverted to their previous religions.
Except within the Papal States, the institution of the Inquisition was abolished in the early 19th century, after the Napoleonic Wars in Europe and after the Spanish American wars of independence in the Americas. The institution survived as part of the Roman Curia, but in 1908 was given the new name of "Supreme Sacred Congregation of the Holy Office". In 1965 it became the Congregation for the Doctrine of the Faith.
Isaac Newton
Newton also built the first practical reflecting telescope and developed a sophisticated theory of colour based on the observation that a prism decomposes white light into the colours of the visible spectrum. Newton's work on light was collected in his highly influential book Opticks, first published in 1704. He also formulated an empirical law of cooling, made the first theoretical calculation of the speed of sound, and introduced the notion of a Newtonian fluid. In addition to his work on calculus, as a mathematician newton contributed to the study of power series, generalised the binomial theorem to non-integer exponents, developed a method for approximating the roots of a function, and classified most of the cubic plane curves. Newton was a fellow of Trinity College and the second Lucasian Professor of Mathematics at the university of Cambridge. He was a devout but unorthodox Christian, who privately rejected the doctrine of the trinity and who, unusually for a member of the Cambridge faculty of the day, refused to take holy orders in the church of England. Beyond his work on the mathematical sciences, Newton dedicated much of his time to the study of alchemy and biblical chronology, but most of his work in those areas remained unpublished until long after his death. Politically and personally tied to the Whig party, Newton served two brief terms as member of Parliament for the University of Cambridge, in 1689 - 90 and 1701 - 02. He was knighted by Queen Anne in 1705 and he spent the last three decades of his life in London, serving as Warden (1696 - 1700) and Master (1700 - 1727) of the royal mint, as well as President of the Royal Society (1703 - 1727).
|
"Nature and Nature's laws lay
hid in night: God said, Let Newton be! and all was light."
- Alexander
Pope
It is hard to understate Isaac Newton's contributions to science. Even Albert Einstein thought of Newton as the greatest scientist ever, and in a recent poll most scientists agreed that is was Newton first, Einstein second, and Maxwell third in importance.
At the time Isaac Newton was alive the Galilean revolution was still being fought by many influential people. Aristocrats, Theologians, Monied Interests, and some Scientists felt that the Galilean revolution would upset the established order (which it did) and they may lose their power, prestige, and perhaps their fortunes. By the time of Newton's death, the Galilean revolution had been won in no small part because of the science of Isaac Newton.
Isaac Newton was a key figure in the "Age of Reason". European politics, philosophy, science and communications were radically reoriented during the course of the "long 18th century" (1685-1815) as part of a movement referred to by its participants as the age of reason, or simply the enlightenment. Enlightenment thinkers in Britain, in France and throughout Europe questioned traditional authority and embraced the notion that humanity could be improved through rational change. The enlightenment produced numerous books, essays, inventions, scientific discoveries, laws, wars, and revolutions. The American and French revolutions were directly inspired by enlightenment ideals and respectively marked the peak of its influence and the beginning of its decline. The enlightenment ultimately gave way to 19th-century romanticism.
Newton's greatest contributions to science were in his Mathematical Principles of Natural Philosophy, (often referred to as simply the Principia), his creation and development of the mathematics of Fluxions (Calculus), and his work on Opticks. Newton's contribution to Gravitational Physics was through his Principia. "Mathematical Principles of Natural Philosophy" is the three-volume work about his laws of motion and universal gravitation that was published in Latin in 1687. It is considered one of the important works of science in history, along with his two other books. His greatest contribution to Atomic Physics was in his work on "Opticks".
Light
In optics, the corpuscular theory of light, arguably set forward by Descartes (1637) states that light is made up of small discrete particles called "corpuscles" (little particles) which travel in a straight line with a finite velocity and possess impetus. This was based on an alternate description of atomism of the time period. This theory cannot explain refraction, diffraction, interference and polarization.
The corpuscular theory was largely developed by Sir Isaac Newton. Newton's theory was predominant for more than 100 years and took precedence over Huygens' wave front theory, partly because of Newton's great prestige. When the corpuscular theory failed to adequately explain the diffraction, interference and polarization of light it was abandoned in favor of Huygens' wave theory. To some extent, Newton's corpuscular (particle) theory of light re-emerged in the 20th century, as light phenomenon is currently explained as particle and wave.
Isaac Newton argued that the geometric nature of reflection and refraction of light could only be explained if light was made of particles, referred to as corpuscles, because waves do not tend to travel in straight lines. Newton sought to disprove Christiaan Huygens' theory that light was made of waves. In a series of experiments concerning physics of light, he concluded that light is made of particles and not waves by having passed a beam of white light through two prisms which were held at such an angle that the light split into a spectrum after passing through the first prism and then was recomposed, back into white light, by the second prism.
Calculus
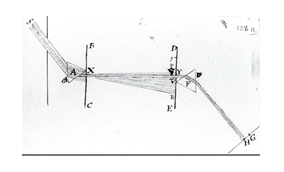
Calculus (from Latin calculus, literally 'small pebble', used for counting and calculations, as on an abacus) is the mathematical study of continuous change, in the same way that geometry is the study of shape and algebra is the study of generalizations of arithmetic operations. It has two major branches, differential calculus (concerning rates of change and slopes of curves), and integral calculus (concerning accumulation of quantities and the areas under and between curves). These two branches are related to each other by the fundamental theorem of calculus. Both branches make use of the fundamental notions of convergence of infinite sequences and infinite series to a well-defined limit. Generally, modern calculus is considered to have been developed in the 17th century by Isaac Newton and Gottfried Wilhelm Leibniz. Today, calculus has widespread uses in science, engineering, and economics.
Calculus is a part of modern mathematics education. A course in calculus is a gateway to other, more advanced courses in mathematics devoted to the study of functions and limits, broadly called mathematical analysis. Calculus has historically been called "the calculus of infinitesimals", or "infinitesimal calculus". The term calculus (plural calculi) is also used for naming specific methods of calculation or notation as well as some theories, such as propositional calculus, Ricci calculus, calculus of variations, lambda calculus, and process calculus.
Newton's Character
To say that Newton was an eccentric character is an understatement. He was an egomaniac, highly suspicious and secretive to the point of paranoia. He knew he was the greatest scientist of his time, and he expected all others to acknowledge his greatness. He would also brook no criticism or critique of his theories and was totally dismissive of all scientific theories with which he disagreed. Easy to offend, Newton was very vindictive to all who offended him. Newton had many confrontations, with both scientist and others, in which his difficult personality led to many problems. Although Newton was the greatest scientist who ever lived, he may also be the greatest pain-in-the-ass scientist that ever lived, although many other scientists throughout history were jockeying for this dubious honor. Here are some personal stories about him.
When Newton was born it was expected that he would not survive. He was underweight and had a sickly appearance, and all were expecting him to pass away within a few days. But he survived and grew up to be the greatest scientist that ever lived. However, he had numerous character flaws due to his upbringing. Several months before Newton was born his father died and he was raised by his single mother and his mother's parents. Although Newton's father was not rich he did have a small estate and the family was not want for food and lodging. Several years later Newton's mother remarried a more prosperous man. Newton was not adopted by his mothers' new husband, and Isaac and his mother's new husband intensely disliked each other. So much so that Newton was sent back to his maternal grandparents to be raised. His maternal grandparents were not that fond of Isaac, but they did get along enough to raise Isaac. As Isaac was a somewhat smaller and robust boy he was not involved in playing with boys of his age. Indeed, the other boys did not like him because Newton was so smart he would point out all the stupidities of the other boys. This led to constant teasing of Isaac Newton, although sometimes Newton was helpful to the other boys. Newton did have a few female friends, but these were of no consequence in his upbringing. Indeed, Newton became a lifelong bachelor and was never known to have any romantic interest or relationships with woman (or men).
Newton's intelligence was very noticeable, as well as his disinclination to get involved in anything other than his observations, studies, and model making of mechanical contrivances. The attempts of Newton's mother's husband to get Newton involved in the business of farming we're also a disaster. With the assistance of some influential people, Newton was admitted to Cambridge University to pursue a religious and academic career. Newton's undergraduate years at Cambridge University we're somewhat difficult. Newton had an inheritance from his natural father, and his mother's husband was a prosperous farmer. However, Newton's mother would not spend any of Newton's inheritance on his academic needs, not to mention her husband wanted nothing to do with Isaac. Therefore, Isaac had to do menial work at Cambridge to support his academic needs. It was also a time at Cambridge in which there was practically no academic supervision, and Newton was free to pursue his studies on whatever he was interested in. Mathematics and science were his interest, but Cambridge did not have much in the way to offer in these subjects. However, Cambridge had a good library and Newton extensively used the library to gain his knowledge in these fields. The newly-created Lucasian chair of mathematics was filled by the professor who had the most knowledge and experience in mathematics. He took a shine to Newton and recognize Newton's genius in mathematics. He tutored Newton in mathematics as much as he could, but he soon realized that Newton's abilities were greater than his own abilities. He was politically connected and used his influence to assure that Newton got his degree and was hired by Cambridge, in what we now know as a graduate position. When he decided to vacate the chair to pursue other personal goals he recommended and pushed for Newton to be named the Lucasian Professor of Mathematics. Newton was appointed to this position and served for over 40 years as Lucasian Professor of Mathematics at Cambridge.
Because of Isaac's circumstances and upbringing, he developed many unpleasant characteristics. He was a loner lacking in social graces. To say that he did not suffer fools gladly was an understatement, as he told people he thought were foolish to go away as they did not know what they were talking about. He was also very paranoid and suspicious of the motives of all those around him. He was concerned that others would steal his ideas and work and take credit for them, a very common occurrence in Newton's time. As a result, he did not often share or publish his findings, but those that he did share and publish where recognized as works of genius. Newton was also a vindictive person, and if you crossed him he often set out to destroy you and often succeeded. The scientist Robert Hooke was often a target of Newton's vindictiveness, as Hooke had a very bad habit of claiming that he had he had already discovered something when another scientist announced their own discoveries. He tried that several times with Newton's discoveries and Newton set out to discredit him, which he did. When Gottfried Leibniz (a brilliant mathematician and scientist from Germany) independently discovered Calculus decades after Newton had developed his Fluxions (his version of Calculus) Newton discovered that Leibowitz had read a short paper that Newton had written giving a brief outline of the method of Fluxions. Newton then claimed that Leibniz had stolen his ideas and set out to destroy Leibniz in order to maintain his priority of discovery. A bitter scientific war of words and letters broke out between the English who defended Newton, and the Germans and others who defended Leibniz. It didn't help Leibniz that he lied about reading the short paper, and therefore Newton was able to discredit Leibniz. The bitterness of that argument lasted for many decades after both had died.
Newton had other peculiarities that in today's world would seem odd, but in his time were not considered out of the ordinary; Alchemy and Biblical Interpretation. Alchemy in Newton's time was much more than the quest to turn base metals into gold. Indeed, those alchemist that engaged in turning metals into gold were looked down upon by the other alchemist who were trying to figure out the nature of the base metals and how they worked. Newton had no interest in turning anything into gold, but he was very interested in discovering how things worked. Newton was deeply interested in all forms of natural sciences and materials science, an interest which would ultimately lead to some of his better-known contributions to science.
During Newton's lifetime, the study of chemistry was still in its infancy, so many of his experimental studies used esoteric language and vague terminology more typically associated with alchemy and occultism. It was not until several decades after Newton's death that experiments of chemistry under the pioneering works of Antoine Lavoisier were conducted, and analytical chemistry, with its associated nomenclature, came to resemble modern chemistry as we know it today. However, Newton's contemporary and fellow Royal Society member, Robert Boyle, had already discovered the basic concepts of modern chemistry and began establishing modern norms of experimental practice and communication in chemistry, information which Newton did not use.
Recently, many new papers on Newton's efforts in alchemy were discovered, and scholars realized that Newton efforts were as much chemistry as it was alchemy. Unfortunately, his Alchemy experiments led into no insights that were useful for Chemistry.
Newton was also a committed believer in God and the Bible, but not of the religious doctrine of the Church. He expended much effort into reading and trying to interpret the underlying meaning of the Bible, looking for hidden insights on nature in the Bible. In reviewing Newton's notes and papers on the Bible modern scholars can confidently say that Newton's work on this subject was complete nonsense.
Christiaan Huygens
 |
Christiaan Huygens, (Dutch: 14 April 1629 - 8 July 1695) was a prominent mathematician and scientist. He is known particularly as an astronomer, physicist, probabilist1 and horologist2. Huygens was a leading scientist of his time. His work included early telescopic studies of the rings of Saturn and the discovery of its moon Titan, the invention of the pendulum clock and other investigations in timekeeping. He published major studies of mechanics and optics (having been one of the most influential proponents of the wave theory of light), and pioneered work on games of chance.
1 Someone who studies probability, a particular branch of mathematics. 2 Someone who makes, or repairs watches or clocks. |
 |
Christiaan Huygens was the first scientist to formulate the wave theory of light, rather than Isaac Newton's formulation that light was a particle. Unfortunately, he made this formulation at the time that Isaac Newton was a demigod in science, therefore most scientists ignored Christiaan Huygens formulation and just accepted Isaac Newton's formulation (an excellent example of why scientists should not defer to authority but search for the truth themselves). Christiaan Huygens was also a leading scientist that helped establish the scientific method and the utilization of mathematics in science, and he made many other valuable contributions to science. His work in mechanics became the basis for some of Isaac Newton's laws of motion. Huygens is also credited as the first theoretical physicist to use formulae in physics. Huygens also invented the first pendulum clock, with an error of less than one minute a day, he went on to refine his clock, ultimately limiting errors to less than ten seconds over twenty-four hours. This invention of the pendulum clock was very important for scientific research, as it allowed for a more precise measurement of time in scientific observations and experiments. His "Opera Reliqua", concerning optics and physics was published in 1728 (posthumously). |
Michael Faraday
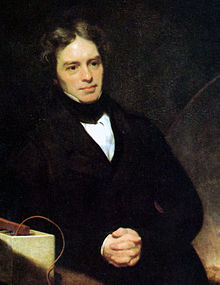 |
Michael
Faraday (English: 22 September 1791 - 25 August
1867) was a scientist who contributed to the study of
electromagnetism and electrochemistry. His main discoveries
include the principles underlying electromagnetic induction,
diamagnetism and electrolysis.
Although Faraday received little formal education, he was one of the most influential scientists in history. It was by his research on the magnetic field around a conductor carrying a direct current that Faraday established the basis for the concept of the electromagnetic field in physics. Faraday also established that magnetism could affect rays of light and that there was an underlying relationship between the two phenomena. He similarly discovered the principles of electromagnetic induction and diamagnetism, and the laws of electrolysis. His inventions of electromagnetic rotary devices formed the foundation of electric motor technology, and it was largely due to his efforts that electricity became practical for use in technology. As a chemist, Faraday discovered benzene, investigated the clathrate hydrate of chlorine, invented an early form of the Bunsen burner and the system of oxidation numbers, and popularised terminology such as "anode", "cathode", "electrode" and "ion". Faraday ultimately became the first and foremost Fullerian professor of chemistry at the Royal Institution, a lifetime position. Faraday was an excellent experimentalist who conveyed his ideas in clear and simple language; his mathematical abilities, however, did not extend as far as trigonometry and were limited to the simplest algebra. James Clerk Maxwell took the work of Faraday and others and summarized it in a set of equations which is accepted as the basis of all modern theories of electromagnetic phenomena. On Faraday's uses of lines of force, Maxwell wrote that they show Faraday "to have been in reality a mathematician of a very high order - one from whom the mathematicians of the future may derive valuable and fertile methods." The si unit of capacitance is named in his honour: The Farad. Albert Einstein kept a picture of Faraday on his study wall, alongside pictures of Isaac Newton and James Clerk Maxwell. Physicist Ernest Rutherford stated, "when we consider the magnitude and extent of his discoveries and their influence on the progress of science and of industry, there is no honour too great to pay to the memory of Faraday, one of the greatest scientific discoverers of all time." |
From the humblest of beginnings to one of the most honored of physicist, Michael Faraday played a key role in the development of atomic physics. Michael Faraday's father was a struggling, ailing, blacksmith who barely supported his family until his death when Michael Faraday was eighteen years old. At thirteen years old Michael Faraday became a newspaper boy and later a bookbinder. As a bookbinder, he became interested in the books he was binding, mainly the "Encyclopedia Britannica" and a self-help text "The Improvement of the Mind". He started attending meetings of the local Philosophical Society, and later the Royal Institution, and became fascinated with physics and chemistry. He taught himself as much physics and chemistry as possible, and at twenty-one, he became an apprentice to Sir Humphry Davy (an important early scientist). This started his stellar career as an experimental physicist and chemist.
Faraday became interested in electrical and magnetic phenomena and performed many brilliant experiments to understand this phenomenon. His discovery of electromagnetic induction led to his invention of the electrical generator and the electrical motor, which altered the history of the world.
His most important contribution to Atomic Physics was his association with James Clerk Maxwell. Faraday's greatest limitation was his inability to master mathematics. This limitation prevented him from converting his experimental results to a theoretical physics. James Clerk Maxwell was the Theoretical Physicist who utilized Faraday's experiments to create the Theory of Electromagnetism, one of the greatest scientific theories of Classical Physics. Even Maxwell acknowledged his debt to Faraday in the development of his Electromagnetic theory.
James Clerk Maxwell
His discoveries helped usher in the era of modern physics, laying the foundation for such fields as special relativity and quantum mechanics. Many physicists regard Maxwell as the 19th-century scientist having the greatest influence on 20th-century physics. His contributions to the science are considered by many to be of the same magnitude as those of Isaac Newton and Albert Einstein. In the millennium poll - a survey of the 100 most prominent physicists - Maxwell was voted the third greatest physicist of all time, behind only Newton and Einstein. On the centenary of Maxwell's birthday, Einstein described Maxwell's work as the "most profound and the most fruitful that physics has experienced since the time of Newton".
|
James Clerk Maxwell was considered the Newton of his time. Every physicist (to this day) have immense respect for him and his work. Every physicist of his time consulted with Maxwell to obtain his advice, opinion, and insight. Maxwell took Faraday's experimental results and created the Theory of Electromagnetism. Maxwell's Theory of Electromagnetism was also the basis for Einstein's Theory of Special Relativity and is the only Classical Physics Theory that survived the Quantum Revolution in physics. Even Newton's theories did not survive (although they are still utilized by engineers of today, but not by physicists). Maxwell also contributed to many other areas of Classical Physics that are too numerous to mention in this short article. The best accolade of Maxwell is the following:

Commemoration of Maxwell's equations at King's College. One of three identical IEEE Milestone Plaques, the others being at Maxwell's birthplace in Edinburgh and the family home at Glenlair.
Maxwell's Character
James Clerk Maxwell was one of the most boring physicists in history. Although he was a genius there were nothing special about his personal life and character. His ego and personality were such that there were no personality clashes or conflicts with the other scientist of his time. Everybody liked and respected Maxwell and sought out his advice and critiques. There was no controversies or scandals in his personal life, and his marriage appeared to be happy and faithful. He was the smart and wise uncle that everybody wished they had.
Ludwig Boltzmann
To quote Planck, "The logarithmic connection between entropy and probability was first stated by Boltzmann in his kinetic theory of gases". This famous formula for entropy is:
|
Ludwig Boltzmann was the first major physicist who became convinced of the reality of atoms. His studies of gases and thermodynamics led him to believe that the only explanation of his results were atoms and molecules of atoms and their interactions. During and prior to his research physicist generally believed that if you could not observe and measure something it did not exist. As atoms and molecules are too small to observe or measure individually most physicists did not believe in them. Ludwig Boltzmann fought that belief in his entire scientific life. He also believed the only way to understand atoms and molecules was through the use of statistical methods and probability, an idea not accepted by physicists of his time. As such he was generally considered out of the mainstream of physics, although his work was very useful for engineers of his time. His other problem is that he could obtain no proof for the physical existence of atoms. Maxwell was also resistant to the idea of atoms, and Max Planck initially opposed the use of statistical methods and probability in physics. Maxwell passed away during this debate and was not able to assist in the resolution of this debate. Planck, in the 1890's decided to thoroughly examine this issue and realized that the only way he could account for the disturbing experimental results regarding light led him to propose that light was composed of a quantum of energy (although he explicitly denied their actual existence), and he utilized a form of statistical analyses (but not in the way Boltzmann had) to describe their behavior. In 1906 Ludwig Boltzmann upon entering old age, with physical ailments and pains, as well as mental exhaustion due to his disagreements with the physics community, took his own life. Shortly thereafter the scientific community recognized that a young Patent Clerk (2nd class), Albert Einstein, had published a paper in 1905 that proved the existence and size of atoms and molecules through Brownian Motion, and he had also proved the existence of Quanta through the Photoelectric Effect (for which Einstein was awarded a Nobel Prize). Ludwig Boltzmann had been vindicated posthumously.
Ludwig Boltzmann Tombstone only notates his famous Entropy equation:


Max Planck
|
Max Planck was the last of the great classical physicist. Thoroughly educated, trained, and knowledgeable in Classical Physics he was recognized in his time as the greatest Classical Physicist after Maxwell. And yet, he became the originator of what we now know as Quantum Physics. In the 1880's and 1890's scientists, through the advancement of instrumentation, began experimenting with light. The results they obtained could not be explained by Classical Physics, and they began proposing hypothesis that did not conform to Classical Physics. Max Planck decided to thoroughly examine these experiments to bring the results inline to classical physics. After many years he determined that they could not be brought in line with classical physics, but if you perceived them in a certain manner the results were explainable. He proposed that light contained a quantum of energy and that if you utilized this quantum in your formulas and calculations the experimental results were understandable. He also used statistical methods (which he was originally was opposed to) to explain lights behavior. Initially, he did not believe in the actual existence of quanta (he explained that it was just a mathematical trick to accomplish a goal). After Einstein proved the existence of Quanta through the Photoelectric Effect Planck was slow to accept the actual existence of quanta. However, by 1914 he did accept their actual existence, and he contributed greatly to the advancement of Quantum Physics. The 1918 Nobel Prize in Physics was awarded to Max Planck "in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta".
Albert Einstein
Between 1895 and 1914, he lived in Switzerland (except for one year in Prague, 1911 - 12), where he received his academic diploma from the Swiss Federal Polytechnic in Z??rich (later the Eidgen??ssische Technische Hochschule, ETH) in 1900. He later taught at that institute as a professor of theoretical physics between 1912 and 1914 before he left for Berlin. In 1901, after being stateless for more than five years, he acquired Swiss citizenship, which he kept for the rest of his life. In 1905, he was awarded a PhD by the university of Z??rich. The same year, his Annus Mirabilis (miracle year), he published four groundbreaking papers, which were to bring him to the notice of the academic world, at the age of 26. He was visiting the United States when Adolf Hitler came to power in 1933 and???being Jewish???did not go back to Germany, where he had been a professor at the Berlin Academy of Sciences. He settled in the United States, becoming an American citizen in 1940. On the eve of World War II, he endorsed a letter to president Franklin D. Roosevelt alerting him to the potential development of "extremely powerful bombs of a new type" and recommending that the U.S. begin similar research. This eventually led to what would become the Manhattan Project. Einstein supported defending the allied forces, but generally denounced the idea of using the newly discovered nuclear fission as a weapon. Later, with the British philosopher Bertrand Russell, he signed the Russell - Einstein manifesto, which highlighted the danger of nuclear weapons. He was affiliated with the Institute for Advanced Study in Princeton, New Jersey, until his death in 1955. Einstein published more than 300 scientific papers along with over 150 non-scientific works. His intellectual achievements and originality have made the word "Einstein" synonymous with "Genius". |
I shall discuss Einstein's contribution to Atomic Physics by presenting a brief outline of the life of Albert Einstein that highlights his means, methods, and achievements.
Childhood
Albert Einstein was born into a middle-class family whose father and Uncle owned an electrical business. Their business was mainly in the manufacture of electrical generators and electrical motors. Although Albert was initially thought to be slow, because of his unwillingness to speak, it soon became a car apparent that he was actually a very bright child. His parents encouraged his education and intellect and as soon as he was old enough they allowed him to play in the research laboratory of his fathers and uncles business. He recalled that time as being a very happy time in his life, and he got to do and see many interesting things in the laboratory. He was especially fond of a compass that his father a given him, and he was fascinated by the fact that no matter where he carried this compass it would always point to the North Pole. This spurred his interest in physics and mathematics in which he excelled. He was always asking questions about why things and how things worked. One of the questions that he asked himself during this time, and which there was no answer, as if he could run alongside a beam of light what the world would look like to him. This question stayed with him throughout his young life, and the answer to this question would be one of his greatest scientific discoveries.
University Years
In 1895, at the age of 16, Einstein took the entrance examinations for the Swiss Federal Polytechnic in Z??rich (later the Eidgen??ssische Technische Hochschule, ETH). He failed to reach the required standard in the general part of the examination but obtained exceptional grades in physics and mathematics. On the advice of the principal of the Polytechnic, he attended the Argovian cantonal school (gymnasium) in Aarau, Switzerland, in 1895 and 1896 to complete his secondary schooling. In September 1896, he passed the Swiss Matura with mostly good grades, including a top grade of 6 in physics and mathematical subjects, on a scale of 1 - 6. At 17, he enrolled in the four-year mathematics and physics teaching diploma program at the Z??rich Polytechnic.
During his Polytechnic student years, Einstein made a few friends, Michele Angelo Besso, Marcel Grossmann, Friedrich Adler, and Mileva Maric with whom he married in 1903. He remained friends with them (except Mileva Maric) throughout their lifetimes.
Einstein was a very poor student at the University, not because of his intellect or abilities, but because he would not behave as a student was expected. In Germany, in Einstein's time, a student was expected to show up for a lecture and which the student would carefully listen to the professor, take good notes, and only ask a question to help clarify what the professor had said. But Einstein was always challenging what the professor said, and this made him very unpopular with the professors. It became so bad that many of his professors made it clear to Einstein that they did not wish him to attend their lectures. Instead Einstein often sat around a coffee table with his friends and discuss the latest papers and experiments in physics, and they would argue as to their meaning. Einstein found this very stimulating as it forced him to think and consider his friend's thoughts on the papers and experiments.
This became a problem for Einstein when he was about to graduate. In order to receive his University degree he had to pass a final exam, an exam which was on what the professors had lectured, which he had not attended. Fortunately, Einstein's friend Marcel Grossmann had attended the lectures and was an excellent note keeper. Einstein borrowed his friend's notes and studiously study them for the next two weeks. He passed the exam with flying colors and then said he probably forgot everything he had studied in the previous two weeks, because he knew it was incorrect.
His next problem was in obtaining employment as a University teacher or research assistant. In Germany at that time, the way you obtained these positions was through a recommendation by your professor. Einstein could not obtain any recommendations by any of his professors. He was therefore unable to obtain a job in his chosen profession. However, his uncle and Marcel Grossmann father were able to obtain a job for Einstein in the Bern Switzerland patent office. As Einstein was newly married and had a child with another on the way he accepted this job to support his family. This unfortunate circumstance, however, turned out to be one of the best things that could happen to him.
Patent Clerk
His position (Patent Clerk 2nd class) at the Swiss patent office in Bern Switzerland (from 1902 - 1909), required him to punctually show up for work we're a stack of patent applications was waiting on his desk for him to review. He was responsible for reviewing the patent applications for any scientific problems or inconsistencies, and if he found any problems or inconsistencies the patent application was rejected. Otherwise, it was passed on to the Patent Clerk (1st class) who reviewed the application to determine if another patent conflicted with it. He was so good at this job that it only took him a few hours to go through the stack of patent applications that was assigned to him. He, therefore, worked on a few of the patent applications then paused to read physics journals and think about what he had read. He would then review a few more patent applications, pause, and read and think ad infinitum throughout the day. This allowed Einstein plenty of time to keep current or what was happening in the world of physics. In 1904 he started concentrating on three questions that were concerning physics; the existence of atoms, the photoelectric effect, and special relativity. In 1905 he had his Annus Mirabilis (Miracle Year), in which he published five papers on these three questions (and a sixth paper in 1906), which answered these questions.
The first the first of these answers had to do with the existence of atoms and molecules. One day Einstein was having a cup of tea in which he placed a lump of sugar. He examined how the sugar dissolved in the tea and postulated that it was a result of the collisions between atoms and molecules, which at that time had not been proven to exist. He abstracted the experiment to deal with pure hot water (a simple molecule), and a pure cold crystal. He was then able to show, mathematically, the motion was a result of the diffusion of an object at a particular rate (known as the mean squared displacement), and that this rate depended upon the number of atoms or molecules in a mole of the fluid in which the object is suspended (Avogadro's number). From this one could determine the size of molecules or atoms. For the first time, a measurable quantity allowed us to probe the atomic realm. It wasn't just the idea, but rather the precision of Einstein's results that many scientists found so convincing. Utilizing his mathematics along with experimentation it would be possible to determine the sizes and masses of all atoms and molecules.
The second answer had to do with the photoelectric effect. The photoelectric effect refers to the emission, or ejection, of electrons from the surface of, generally, a metal in response to incident light. Max Planck at the end of the 1890's had postulated the existence of energy quanta as the reason for Black Body Radiation, but his explanation had several unresolved problems (mainly the Photoelectric Effect and the Ultraviolet Catastrophe). There was also the problem that in most experiments light generally behaved as a wave, but in some experiments, light was behaving as if it were a particle. Also, Planck did not believe that quanta actually existed, but we're only a mathematical trick to explain the phenomena. Albert Einstein, however, believed they actually existed, and that light was much stranger than anyone had imagined. Einstein showed that light had a dual nature - sometimes it behaved as a wave, and sometimes it behaved as a particle. Depending on the experiment you were conducting you were either measuring the wave nature of light, or the particle nature of light. He described light as composed of discrete quanta (now called photons) as opposed to continuous waves, but that light traveled in a wave-like manner but interacted with other matter in a particle fashion. This explanation resolved all the problems and discrepancies of Black Body Radiation, the Photoelectric Effect, and the Ultraviolet Catastrophe. It was for this theory that Einstein was awarded his only Nobel Prize in 1921.
The third answer had to do with his question, as a young man, of what the world would like look like if you could run next to a light beam. Einstein was very punctual in arriving at the patent office, as was required at that time. He took the same trolley every day, at the same time, from his apartment to the patent office, and he even sat in the same seat each day. As this trolley pass by the Town Center on the way to the patent office Einstein was looking forward in his trolley ride to the center of town, and he often looked at the clock tower. After passing the Clock Tower he would start looking at the patent office and think about what he was going to do that day. One day, due to a family issue, he missed his regular trolley and had to catch the next trolley. This time he was facing away from the Clock Tower when he approached the town center, and when he passed the Clock Tower he was facing toward the clock. He began to wonder what's a clock would show if he was riding on a beam of light instead of a trolley. He realized that as he traveled faster and faster it would take the next beam of light, that showed the next minute, longer to overtake the beam of light he was traveling on. He also realized that if he was traveling at the speed of light the next beam of light would never overtake him, and for all intents and purposes time stood still for a beam of light. He then looked over his shoulders and realized he was traveling so fast that everything to the right and left of him looked foreshorten. He then looked over his back and realized that the entire universe would collapse in front of him as he was traveling toward the speed of light, eventually into a single point in the direction of travel when he reached the speed of light. He also realized that as he was traveling faster toward the speed of light that it would take more and more energy to speed him up, and that it would take all the energy in the universe to get him to the speed of light. This was an astounding insight and Einstein was very excited about it. He rushed to his desk in the patent office and immediately went through a stack of patent applications and then stopped reviewing the patent applications and started doing the mathematics of his insight. By noon he had the answer mathematically and scientifically, and he stated it was one of the most exhilarating moments of his life that he knew something about the universe that no one else knew. Upon further work on this Insight he realized that not only did Time, Length, and Mass change relative to your speed, but that different observers at different places traveling at different speeds could look at the same phenomena and report back different observations of what they had seen because of this effect. He named this phenomenon Relativity (which we now know as Special Relativity to distinguish it from General Relativity which he developed a few years later). His science and math were based on Maxwell's equations of electrodynamics, and Maxwell's equations where one of the few things that survived from classical physics because of this. He also realized that as a result of this phenomena mass and energy were equivalent, which resulted in his famous equation: E=mc2.
Einstein continued to publish papers on the impacts of these answers, but he was generally ignored. After all, who could take seriously an unknown, unaffiliated, physicist from Bern Switzerland (he took pains to hide his occupation as a patent clerk)? Max Planck, however, took an interest in his Special Relativity, and other prominent physicist started reviewing his work. In 1907 several light experiments were performed in which only Einstein's Photoelectric Theory could explain the results. More physicist started taking him seriously, and by 1909 it was recognized that he was a genius (which allowed him to leave the patent office and become an associate professor of Theoretical Physicist at the University of Z??rich). His previous papers were then studied and incorporated into the new field of Quantum Physics.
Theoretical Physicist
By 1908, Einstein was recognized as a leading scientist and was appointed lecturer at the University of Bern in Switzerland. The following year, after giving a lecture on electrodynamics and the relativity principle at the University of Z??rich in Switzerland, he was recommended to the faculty at the University of Z??rich for a newly created professorship in theoretical physics. Einstein was appointed an associate professor in 1909, and Einstein became a full professor at the German Charles-Ferdinand University in Prague Czechoslovakia in April 1911. Thereafter, he held several positions as a Theoretical Physicist at notable institutions throughout Europe and the United States. By the time Einstein became a Theoretical Professor, he had become interested in the problem of Gravity. Newton's Theory of Universal Gravity had held sway for over two hundred years, but it never explained what gravity was, only how it worked (a point that Newton freely acknowledged). In addition, there were also some cracks that were beginning to appear with Newtonian gravity (mainly the Precession of the Perihelion of Mercury's orbit). Einstein spent the next several years working on gravity (a period that he called the most arduous of his life). As a result of these efforts, he formulated the Theory of General Relativity which explained all of gravity, and which also incorporated his Special Relativity theory. General Relativity has since withstood all observations and experiments to become a bedrock of modern physics.
Einstein eventually returned to Quantum Physics but was dismayed by the course it had taken under Neils Bohr. At that point, he became a critic who raised more objections than contributions to Quantum Physics (but his objections were profound and provoked much discussion and reassessment which led to better Quantum Physics). The last few decades of Einstein's life were spent trying to reconcile Quantum Theory and General Relativity, to no avail. We are still, to this day, trying to reconcile Quantum Physics with General Relativity.
Einstein's Character
If Isaac Newton was the greatest pain-in-the-ass scientists who ever lived than Albert Einstein, with the possible exception of James Clerk Maxwell, was the most beloved scientist in history. Born a German Jew he grew up with a natural dislike of authority and militarism. So much so that he revoked his German citizenship as a young man and became a Swiss citizen. When he moved to the United States to escape Nazism he obtained dual citizenship as an American and Swiss citizen. He was a lifelong pacifist, except when he recognized the existential threat of Nazism and Fascism and supported the American entry into World War II. Upon realizing that the German scientists had the knowledge and capability to construct an atomic bomb he urged President Franklin D. Roosevelt to develop an American atomic bomb. He was appalled when the atomic bomb was used on Japan, and he became a proponent of Nuclear Disarmament. Essentially non-religious in his younger life he became more religious later in life, and he also became a supporter of the Zionists movement. Offered the Presidency of Israel he turned it down, as he knew he would be a disaster as a political leader. Einstein was also an amateur violinist, at which he was not very good, and often used his violin playing to entertain his friends and help him concentrate his thoughts.
During his Polytechnic student years, Einstein made a few good friends, Michele Angelo Besso, Marcel Grossmann and Friedrich Adler, and he remained friends with them throughout their lifetimes. He also utilized these friends' assistance in developing his scientific theories. During the course of his scientific career, he also made other good friends with whom he remained in contact throughout their lifetimes.
Einstein's romantic and sexual life was complicated. He had a pre-university romance, a marriage, divorce, and then remarriage. He was known for philandering as his first marriage failed, as well as philandering during his second marriage.
When Einstein was a young man he was considered handsome and a good prospect for marriage. He had a brief relationship with Marie Winteler, a local young lady. He soon broke up with her (who Einstein believed had become clingy) and wrote a letter to her mother explaining his reasoning. In this letter, an 18-year-old Einstein who had been invited to stay at the family's country house. Einstein declines the invitation, citing that he would not want to lead on young Marie any more than he already has. It is a remarkably discerning and introspective letter, which illustrates not only the emotional and social maturity of Einstein, but also his becoming self-aware that physics is not merely something he wants to do, it is something he must do.
Upon entering the University he met Mileva Maric, and intelligent young physics student, with whom he soon fell in love with. Mileva was not very attractive, but Albert stated that it was her mind that he was most attracted too. The relationship did not have the approval of Albert's or Mileva's mother, but they eventually accepted it. They married after Einstein obtained a position at the Swiss Patent Office and he could afford to support both of them. Soon after, Albert and Mileva started a family. Albert's three children were from his relationship with Mileva, his daughter Lieserl being born a year before they married, and who was given up for adoption before the marriage.
Lieserl Einstein (born January 1902 - last mentioned in 1903; possible date of death, 18 September 1903) was the first child of Mileva Maric and Albert Einstein. According to the correspondence between her parents, Lieserl was born in January 1902, a year before her parents married, in Novi Sad, Vojvodina, present-day Serbia, and was cared for by Mileva and her mother for a short time while Einstein worked in Switzerland. When Mileva joined Albert in Switzerland it was without the child, as Lieserl had been given up for adoption to a good friend of Mileva. It was rumored that Lieserl was a sickly child and she died at an early age. Lieserl's existence was unknown to biographers until 1986, when a batch of letters between Albert and Mileva was discovered by Hans Albert Einstein's daughter Evelyn. It is reported (and probably true) that Einstein never saw his daughter.
Hans Albert Einstein (May 14, 1904 - July 26, 1973) was a Swiss-American engineer and educator, the second child and first son of Albert Einstein and Mileva Maric. Hans A. Einstein was a professor of Hydraulic Engineering at the University of California, Berkeley, and was recognized for his research on sediment transport.
Eduard Einstein (28 July 1910 - 25 October 1965) was born in Z??rich, Switzerland, the second son of physicist Albert Einstein from his first wife Mileva Maric. Eduard was a good student and had musical talent. After high school, he started to study medicine to become a psychiatrist, but by the age of twenty, he was diagnosed with schizophrenia. He was institutionalized two years later for the first of several times. Biographers of his father have speculated that the drugs and "cures" of the time damaged rather than aided the young Eduard. His brother Hans Albert Einstein believed that his memory and cognitive abilities were damaged by electroconvulsive therapy treatments. After suffering a breakdown, Eduard told his father that he hated him. Albert Einstein emigrated to the United States from Germany in 1933 after the rise of the Nazi German government, and never saw Eduard again. His mother cared for Eduard until she died in 1948. From then on Eduard lived most of the time at the psychiatric clinic Burgh??lzli in Zurich, where he died of a stroke in 1965 at the age of 55.
It is known that Einstein was a good father to Hans and Eduard during their younger lives. However, the strain of the breakup and divorce with their mother put a strain on his relationships with his sons, and it would be a contentious relationship during this period. Einstein tried to maintain a harmonious relationship with his sons but often failed. During Einstein's later life Hans and Albert had a reconciliation, but Eduard and Albert never reconciled.
The divorce with Mileva was contentious and somewhat bitter. Mileva never obtained her university diploma (she failed the final exam thrice), and therefore never had a career as a physicist which she had dreamed of since adolescence. As Albert's fame grew her resentment grew and this put a great strain on their marriage. The loss of their daughter Lieserl also played a part in the dissolving marriage. Einstein was also a difficult man to live with. He often brought his work home with him. When he did this, he demanded quiet and no interruptions of his thoughts or calculations. He was also not interested in the day-to-day activities of running a household. He expected that Mileva would handle all these responsibilities. Then, as the marriage began to dissolved Einstein started philandering with his secretary followed by his cousin Elsa. At this point, the marriage was effectively over. The divorce took several years to finalize with contentious negotiations on the financial support for Mileva and Einstein's sons.
After his divorce from Mileva, Albert realized he needed a wife to take care of his earthly needs as he pursued his scientific endeavors. His cousin Elsa Einstein (with whom he started having sexual trysts) was always smitten with Albert, and she gladly stepped into this role. They were affectionate with each other, but probably not in love, but they decided to marry. As Albert was gaining fame in the physics world he had the opportunity, and took advantage of it, to engage in sexual trysts with many adoring women. After gaining world fame the opportunities to philander became much greater to which Albert indulged himself. As long as the relationship was not serious Elsa didn't seem to mind, as she was enthralled at being the wife of the great Albert Einstein. There was also the social status, wealth, security and travel of being Albert Einstein's wife.
For her reasons, Elsa stayed with Einstein despite his flaws and explained her views about him in a letter: "Such a genius should be irreproachable in every respect. But nature does not behave this way, where she gives extravagantly, she takes away extravagantly."
But it's not to say Einstein didn't have a conscience about his personal failures. Writing to a young gentleman, Einstein admitted as much; "What I admire in your father is that, for his whole life, he stayed with only one woman. This is a project in which I grossly failed, twice."
For all of Einstein's immortalized genius, his love life proved he was very much a human tethered to Earth.
Albert Einstein Quotes:
Albert Einstein was one of the most quotable scientists, not only on science but life itself. Below are some of my favorite Einstein quotes.
|
"A man should look for what is, and not for what he thinks should be." "All that is valuable in human society depends upon the opportunity for development accorded the individual." "Any man who reads too much and uses his own brain too little falls into lazy habits of thinking." "Anyone who has never made a mistake has never tried anything new." "Common sense is nothing more than a deposit of prejudices laid down by the mind before you reach eighteen." "Do not worry about your difficulties in mathematics, I can assure you that mine are all greater." "Education is what remains after one has forgotten what one has learned in school." "Falling in love is not at all the most stupid thing that people do??? but gravitation cannot be held responsible for it." "Great spirits have always encountered violent opposition from mediocre minds. The mediocre mind is incapable of understanding the man who refuses to bow blindly to conventional prejudices and chooses instead to express his opinions courageously and honestly." "Human knowledge and skills alone cannot lead humanity to a happy and dignified life. Humanity has every reason to place the proclaimers of high moral standards and values above the discoverers of objective truth." "I have no special talent. I am only passionately curious." "I speak to everyone in the same way, whether he is the garbage man or the president of the university." "I think and think for months and years. Ninety-nine times, the conclusion is false. The hundredth time I am right." "I very rarely think in words at all. A thought comes, and I may try to express it in words afterwards." "I, at any rate, am convinced that He (God) does not throw dice." "If you can't explain it simply, you don't understand it well enough." "Imagination is everything. It is the preview of life's coming attractions." "Imagination is more important than knowledge. For knowledge is limited, whereas imagination embraces the entire world, stimulating progress, giving birth to evolution." "In order to form an immaculate member of a flock of sheep, one must, above all, be a sheep." "Information is not knowledge." "Insanity: doing the same thing over and over again and expecting different results." "It is the supreme art of the teacher to awaken joy in creative expression and knowledge." "It's not that I'm so smart, it's just that I stay with problems longer." "Learn from yesterday, live for today, hope for tomorrow. The important thing is not to stop questioning." "Life is like riding a bicycle. To keep your balance you must keep moving." |
"Memory is deceptive because it is colored by today's events." "No amount of experimentation can ever prove me right; a single experiment can prove me wrong." "Once we accept our limits, we go beyond them." "Only a life lived for others is a life worthwhile." "Pure mathematics is, in its way, the poetry of logical ideas." "Reality is merely an illusion, albeit a very persistent one." "Reality is the real business of physics." "Science without religion is lame, religion without science is blind." "Small is the number of people who see with their eyes and think with their minds." "The difference between genius and stupidity is that genius has its limits." "The important thing is not to stop questioning. Curiosity has its own reason for existing." "The monotony and solitude of a quiet life stimulates the creative mind." "The most beautiful experience we can have is the mysterious. It is the fundamental emotion that stands at the cradle of true art and true science." "The most incomprehensible thing about the world is that it is comprehensible." "The only source of knowledge is experience." "The only way to escape the corruptible effect of praise is to go on working." "The secret to creativity is knowing how to hide your sources." "The true sign of intelligence is not knowledge but imagination." "The value of a man should be seen in what he gives and not in what he is able to receive." "To punish me for my contempt for authority, fate made me an authority myself." "To raise new questions, new possibilities, to regard old problems from a new angle, requires creative imagination and marks real advance in science." "Truth is what stands the test of experience." "Try not to become a man of success, but rather try to become a man of value. " "Two things are infinite: the universe and human stupidity; and I'm not sure about the universe." "We cannot solve our problems with the same thinking we used when we created them." "Weakness of attitude becomes weakness of character." "Whoever is careless with the truth in small matters cannot be trusted with important matters." "You ask me if I keep a notebook to record my great ideas. I've only ever had one." |
The Nobel Prize in Physics in 1921 was awarded to Albert Einstein "for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect".
Neils Bohr
During the 1930s, Bohr helped refugees from Nazism. After Denmark was occupied by the Germans, he had a famous meeting with Heisenberg, who had become the head of the German nuclear weapon project. In September 1943, word reached Bohr that he was about to be arrested by the Germans, and he fled to Sweden. From there, he was flown to Britain, where he joined the British Tube Alloys Nuclear Weapons Project, and was part of the British mission to the Manhattan project. After the war, Bohr called for international cooperation on nuclear energy. He was involved with the establishment of CERN and the research establishment Ris?? of the Danish Atomic Energy Commission and became the first chairman of the Nordic institute for theoretical physics in 1957. |
While Max Plank and Albert Einstein started the quantum revolution in physics Neils Bohr was primarily responsible for advancing quantum theory. He seized the initiative and gathered and encouraged like-minded scientists in expanding and improving the quantum theory. Other scientists started performing experiments and developing other interpretations of quantum theory.
The Nobel Prize in Physics in 1922 was awarded o Niels Henrik David Bohr "for his services in the investigation of the structure of atoms and of the radiation emanating from them".
Atomic Model
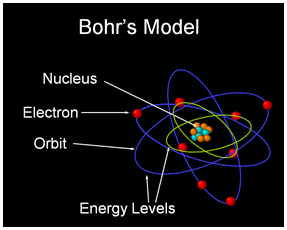 |
Neils Bohr proposed a model of the atom that solved many problems of his time but also had a few problems of its own. His model had a positive charged nucleus (composed of protons and neutrons), and negatively charged electrons that revolved around the nucleus in fixed orbits. Each orbit was only allowed to have a maximum number of electrons (energy levels). This is how most people think about atoms today, but it is an incorrect model. The correct atomic model of Modern Quantum Theory is so intricate that non-physicist have difficulty understanding the model (so we shall ignore it as beyond the scope of this paper). |
There were other models proposed that also had problems associated with them. So much so that it became necessary to convene a conference to try to reach agreement on a core interpretation of quantum theory. The Copenhagen interpretation (named after the city in which the conference was held) of quantum theory was the result. The Copenhagen interpretation starts from a paradox. Any experiment in physics, whether it refers to the phenomena of daily life or to atomic events, is to be described in the terms of classical physics. The concepts of classical physics form the language by which we describe the arrangement of our experiments and state the results. We cannot and should not replace these concepts with any others. Still, the application of these concepts is limited by the relations of uncertainty. We must keep in mind this limited range of applicability of the classical concepts while using them, but we cannot and should not try to improve them.
For a better understanding of this paradox, it is useful to compare the procedure for the theoretical interpretation of an experiment in classical physics and in quantum theory. In Newton's mechanics, for instance, we may start by measuring the position and the velocity of the planet whose motion we are going to study. The result of the observation is translated into mathematics by deriving numbers for the coordinates and the momenta of the planet from the observation. Then the equations of motion are used to derive from these values of the coordinates and momenta at a given time the values of these coordinates or any other properties of the system at a later time, and in this way, the astronomer can predict the properties of the planet at a later time. He can, for instance, predict the exact time for an eclipse of the moon.
In quantum theory, the procedure is slightly different. We could, for instance, be interested in the motion of an electron through a cloud chamber and could determine by some kind of observation the initial position and velocity of the electron. But this determination will not be accurate; it will at least contain the inaccuracies following from the uncertainty relations and will probably contain still larger errors due to the difficulty of the experiment. It is the first of these inaccuracies which allows us to translate the result of the observation into the mathematical scheme of quantum theory. A probability function is written down which represents the experimental situation at the time of the measurement, including even the possible errors of the measurement.
This probability function represents a mixture of two things, partly a fact and partly our knowledge of a fact. It represents a fact in so far as it assigns at the initial time the probability unity (i.e., Complete certainty) to the initial situation: The electron moving with the observed velocity at the observed position; "observed" means observed within the accuracy of the experiment. It represents our knowledge in so far as another observer could perhaps know the position of the electron more accurately. The error in the experiment does at least to some extent not represent a property of the electron but a deficiency in our knowledge of the electron. Also, this deficiency of knowledge is expressed in the probability function.
Solvay Conference
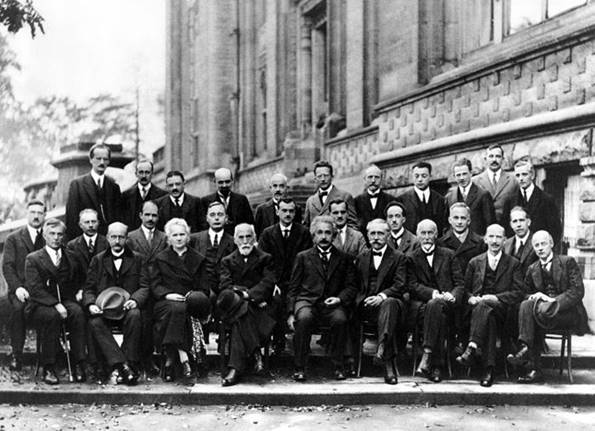
Solvay conference, 1927.
At the 1927 Solvay conference on physics entitled "Electrons and Photons," Niels Bohr and Werner Heisenberg consolidated their Copenhagen view as a "complete" picture of quantum physics, despite the fact that they could not, or would not, visualize or otherwise explain exactly what is going on in the microscopic world of "quantum reality."
Bohr seemed to deny the existence of an "objective reality," but clearly knew and said that the physical world is largely independent of human observations. In classical physics, the physical world is assumed to be completely independent of the act of observing the world. In quantum physics, Heisenberg said that the result of an experiment depends on the free choice of the experimenter as to what to measure. The quantum world of photons and electrons might look like waves or look like particles depending on what we look for, rather than what they "are" as "things in themselves."
Copenhageners were proud of their limited ability to know. Bohr said:
"There is no quantum world. There is only an abstract quantum physical description. It is wrong to think that the task of physics is to find out how nature is. Physics concerns what we can say about nature."
The implications of the quantum theory are so profound that even its creators, such as Planck and Einstein, found it difficult to accept and wrestled with its concepts all their lives. Skeptical physicists devised ways to avoid the apparent contradictions, and these proposals have led to experimental tests. All of the tests performed so far have confirmed the predictions of quantum theory, although its laws are subject to different physical interpretations.
Max Born
 |
Max
Born (German 11 December 1882 - 5 January 1970) was a
German-Jewish physicist and mathematician who was
instrumental in the development of quantum mechanics. He
also made contributions to solid-state physics and optics
and supervised the work of a number of notable physicists in
the 1920s and 1930s. Born won the 1954 Nobel Prize in
Physics for his "fundamental research in quantum mechanics,
especially in the statistical interpretation of the wave
function". Born entered the University of G?ttingen in 1904,
where he found the three renowned mathematicians Felix
Klein, David Hilbert, and Hermann Minkowski. He wrote his
Ph.D. thesis on the subject of "Stability of Elastica in a
Plane and Space", winning the University's Philosophy
Faculty Prize. In 1905, he began researching special
relativity with Minkowski, and subsequently wrote his
habilitation thesis on the Thomson model of the atom. A
chance meeting with Fritz Haber in Berlin in 1918 led to
discussion of the manner in which an ionic compound is
formed when a metal reacts with a halogen, which is today
known as the Born - Haber cycle.
In the First World War, after originally being placed as a radio operator, he was moved to research duties regarding sound ranging due to his specialist knowledge. In 1921, Born returned to G?ttingen, arranging another chair for his long-time friend and colleague James Franck. Under Born, G?ttingen became one of the world's foremost centres for physics. In 1925, Born and Werner Heisenberg formulated the matrix mechanics representation of quantum mechanics. The following year, he formulated the now-standard interpretation of the probability density function for ??*?? in the Schrodinger equation, for which he was awarded the Nobel Prize in 1954. His influence extended far beyond his own research. Max Delbr??ck, Siegfried Fl??gge, Friedrich Hund, Pascual Jordan, Maria Goeppert-Mayer, Lothar Wolfgang Nordheim, Robert Oppenheimer, and Victor Weisskopf all received their Ph.D. degrees under Born at G?ttingen, and his assistants included Enrico Fermi, Werner Heisenberg, Gerhard Herzberg, Friedrich Hund, Pascual Jordan, Wolfgang Pauli, L??on Rosenfeld, Edward Teller, and Eugene Wigner. In January 1933, the Nazi Party came to power in Germany, and Born, who was Jewish, was suspended from his professorship at the University of G?ttingen. He emigrated to the United Kingdom, where he took a job at St John's College, Cambridge, and wrote a popular science book, The Restless Universe, as well as Atomic Physics, which soon became a standard textbook. In October 1936, he became the Tait Professor of Natural Philosophy at the University of Edinburgh, where, working with German-born assistants E. Walter Kellermann and Klaus Fuchs, he continued his research into physics. Born became a naturalised British subject on 31 August 1939, one day before World War II broke out in Europe. He remained at Edinburgh until 1952. He retired to Bad Pyrmont, in West Germany, and died in hospital in G?ttingen on 5 January 1970. Max Born became interested in the works of Albert Einstein and by the end of 1913 Born had published 27 papers, including important work on relativity and the dynamics of crystal lattices. After Niels Bohr published his works on the model of the atom Born essentially expanded upon and solidified the work of Niels Bohr. In 1923 Werner Heisenberg became Born's assistant, and in 1925 Born and Heisenberg formulated the matrix mechanics representation of quantum mechanics. Max Born won the The Nobel Prize in Physics in 1954 "for his fundamental research in quantum mechanics, especially for his statistical interpretation of the wave function". |
Werner Heisenberg
 |
Werner Karl Heisenberg (German: 5 December 1901 - 1 February 1976) was a theoretical physicist and one of the key pioneers of quantum mechanics. He published his work in 1925 in a breakthrough paper. In the subsequent series of papers with Max Born and Pascual Jordan, during the same year, this matrix formulation of quantum mechanics was substantially elaborated. He is known for the Heisenberg uncertainty principle, which he published in 1927. Heisenberg was awarded the Nobel Prize in physics for 1932 "for the creation of quantum mechanics". He also made important contributions to the theories of the hydrodynamics of turbulent flows, the atomic nucleus, ferromagnetism, cosmic rays, and subatomic particles, and he was instrumental in planning the first west German nuclear reactor at Karlsruhe, together with a research reactor in Munich, in 1957. He was a principal scientist in the Nazi German nuclear weapon project during World War II. He traveled to occupied Copenhagen where he met and discussed the German project with Niels Bohr. Following World War II, he was appointed director of the Kaiser Wilhelm institute for Physics, which soon thereafter was renamed the Max Planck institute for Physics. He was director of the institute until it was moved to Munich in 1958, when it was expanded and renamed the Max Planck institute for Physics and Astrophysics. Heisenberg was also president of the German research council, chairman of the commission for atomic physics, chairman of the nuclear physics working group, and president of the Alexander von Humboldt Foundation. |
The Uncertainty Principle, also called Heisenberg Uncertainty Principle or Indeterminacy Principle, is a statement articulated in 1927 by the German physicist Werner Heisenberg. It is one of the most famous (and probably misunderstood) ideas in physics. It tells us that there is a fuzziness in nature, a fundamental limit to what we can know about the behavior of quantum particles and, therefore, the smallest scales of nature. Of these scales, the most we can hope for is to calculate probabilities for where things are and how they will behave. It tells us that the position and the velocity of an object cannot both be measured exactly, at the same time, even in theory. The very concepts of exact position and exact velocity together, in fact, have no meaning in nature. Unlike Isaac Newton's clockwork universe, where everything follows clear-cut laws on how to move and prediction is easy if you know the starting conditions, the uncertainty principle enshrines a level of fuzziness into quantum theory.
Ordinary experience provides no clue of this principle. It is easy to measure both the position and the velocity of, say, an airplane because the uncertainties implied by this principle for ordinary objects are too small to be observed. The complete rule stipulates that the product of the uncertainties in position and velocity is equal to or greater than a tiny physical quantity. Only for the exceedingly small masses of atoms and subatomic particles does the product of the uncertainties become significant. Werner Heisenberg's simple idea tells us why atoms don't implode, how the sun manages to shine and, strangely, that the vacuum of space is not actually empty.
This is analogous to the blind men and the elephant parable:
"A group of blind men heard that a strange animal, called an elephant, had been brought to the town, but none of them were aware of its shape and form. Out of curiosity, they said: "We must inspect and know it by touch, of which we are capable". So, they sought it out, and when they found it they groped about it. In the case of the first person, whose hand landed on the trunk, said: "This being is like a thick snake". For another one whose hand reached its ear, it seemed like a kind of fan. As for another person, whose hand was upon its leg, said, the elephant is a pillar like a tree-trunk. The blind man who placed his hand upon its side said, "elephant is a wall". Another who felt its tail described it as a rope. The last felt its tusk, stating the elephant is that which is hard, smooth and like a spear."
Quantum physicist is like the blind men, only they combined their observations to try to determine the real nature of the elephant (atomic & sub-atomic universe), but they cannot observe the actual nature of the atomic & sub-atomic universe.
Erwin Schrodinger
 |
Erwin Rudolf Josef Alexander Schrodinger (Austrian: 12 August 1887 - 4 January 1961), sometimes written as Erwin Schrodinger, was a Nobel prize-winning physicist who developed a number of fundamental results in the field of quantum theory, which formed the basis of wave mechanics: He formulated the wave equation (stationary and time-dependent Schrodinger equation) and revealed the identity of his development of the formalism and matrix mechanics. Schrodinger proposed an original interpretation of the physical meaning of the wave function. In addition, he was the author of many works in various fields of physics: statistical mechanics and thermodynamics, physics of dielectrics, colour theory, electrodynamics, general relativity, and cosmology, and he made several attempts to construct a unified field theory. In his book what is life? Schrodinger addressed the problems of genetics, looking at the phenomenon of life from the point of view of physics. He paid great attention to the philosophical aspects of science, ancient and oriental philosophical concepts, ethics, and religion. He also wrote on philosophy and theoretical biology. He is also known for his "Schrodinger's Cat" thought-experiment. Erwin Schrodinger is best known for his work regarding quantum theory, particularly about his thought experiment involving a cat in order to explain the flawed interpretation of quantum superposition. |
The Copenhagen Interpretation of quantum mechanics essentially states that an object in a physical system can simultaneously exist in all possible configurations, but observing the system forces the system to collapse and forces the object into just one of those possible states. Schrodinger disagreed with this interpretation.
So, what does this have to do with cats? Schrodinger wanted people to imagine that a cat, a container of poison, a Geiger counter, radioactive material, and a hammer were inside of a sealed container. The amount of radioactive material was minuscule enough that it only had a 50/50 shot of being detected over the course of an hour. If the Geiger counter detected radiation, the hammer would smash the poison, killing the cat. Until someone opened the container and observed the system, it was impossible to predict if the cat's outcome. Thus, until the system collapsed into one configuration, the cat would exist in some superposition zombie state of being both alive and dead.
Of course, Schrodinger claimed, that was ridiculous. Quantum superposition could not work with large objects such as cats, because it is impossible for an organism to be simultaneously alive and dead. Thus, he reasoned that the Copenhagen Interpretation must be inherently flawed. While many people incorrectly assume Schrodinger supported the premise behind the thought experiment, he really didn't. His entire point was that it was impossible.
While it is true that modern experiments have revealed that while quantum superposition does work for tiny things like electrons, larger objects must be regarded differently.
Paul Dirac
 |
Paul Adrien Maurice Dirac (English: 8 August 1902 - 20 October 1984) was a theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics. He was the Lucasian professor of mathematics at the University of Cambridge, a member of the Center for Theoretical Studies, University of Miami, and spent the last decade of his life at Florida State University. Among other discoveries, he formulated the Dirac equation which describes the behaviour of fermions and predicted the existence of antimatter. Dirac shared the 1933 Nobel Prize in physics with Erwin Schrodinger "for the discovery of new productive forms of atomic theory". He also made significant contributions to the reconciliation of general relativity with quantum mechanics. Dirac was regarded by his friends and colleagues as unusual in character. In a 1926 letter to Paul Ehrenfest, Albert Einstein wrote of Dirac, "this balancing on the dizzying path between genius and madness is awful". He is regarded as one of the most significant physicists of the 20th century. |
From 1925 to 1927 three equivalent new versions of quantum mechanics were proposed that extended the previous Bohr-Sommerfeld theory of quantum mechanics, cured it of its main difficulties, and produced an entirely novel view of the microworld. These new theories were the matrix mechanics of Werner Heisenberg (1901 - 1976), the wave mechanics of Erwin Schrodinger (1887 - 1951), and the transformation theory of Paul A. M. Dirac (1902 - 1984), the last being a more general version that includes both of the other versions. It is Daric's equations that are utilized today, as it covers all the interpretations.
The Nobel Prize in Physics in 1932 was awarded to Werner Karl Heisenberg for "for the creation of quantum mechanics, the application of which has, inter alia, led to the discovery of the allotropic forms of hydrogen", while The Nobel Prize in Physics in 1933 was awarded to both Erwin Schr??dinger and Paul Adrien Maurice Dirac for "for the discovery of new productive forms of atomic theory".
Modern Quantum Theory
After World War II, and the development of the Atomic Bomb, government's involvement in Atomic Physics increased dramatically. Hundreds of important scientists and thousands of supporting scientists contributed to the development of Quantum Physics, unlike the previous times where it was dozens of important scientists and hundreds of supporting scientists. The advancement of Atomic Physics grew dramatically as a result. For more information I would direct you to the Wikipedia article "History of quantum field theory". The most important development was the development of the "Standard Model" of quantum particles. With more and better instrumentation atomic physicist began discovering dozens then hundreds of sub-atomic particles. So much so that atomic physicist began searching for a simpler explanation of atomic particles. The result was the development of the "Standard Model" of quantum physics. By combining the various quantum particles, in various combinations, you could account for all the various sub-atomic particles.
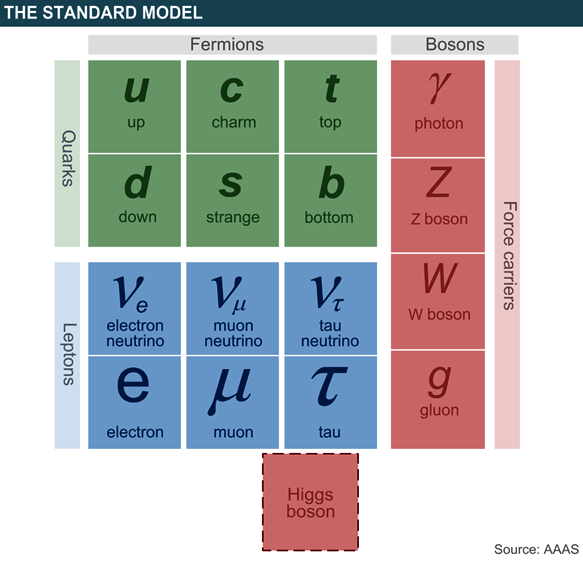
Fermions were composed of mass (Quark particles) and energy (Lepton particles). Boson's were force particles that bound the Fermions.
They then discovered the ways that these quantum particles could be combined through three properties of a quantum particle; Mass, Charge, and Spin. Complex mathematical formulas were developed that limited the acceptable ways of combining the elementary particles.
Standard Model of Particle Physics
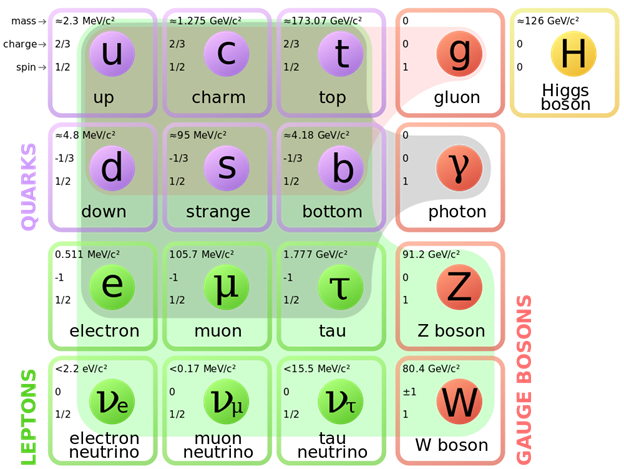
This is the current model of Quantum Physics which all quantum physicist utilizes in their experiments, hypotheses, and theories. For more information on the Standard Model I would direct you to the Wikipedia article on this subject.
Additional Thoughts
How did all this happen?
Where and how were all these particles created? The answer is in the initial creation of the Universe via the "Big Bang".
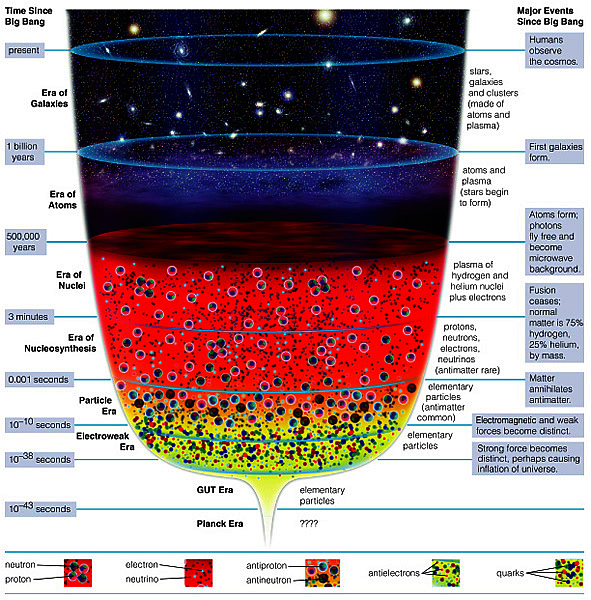
In the very beginning, all the matter in the Universe was compressed into a space the size of a subatomic particle. The temperature and density were so unimaginably high, that the common laws of physics were inapplicable. Scientists are still looking for theories and laws that could help explain this and what caused the huge explosion called the Big Bang. During this explosion, the first subatomic particles that makeup matter and energy were created. Space and time were born, and the cosmic clock started to run. At first, the Universe was extremely hot and dense with stuff flying in all directions with unimaginable speed. Scientists call this the inflationary epoch. In less than a millionth of a second, the Universe expanded from the size of a subatomic particle to a trillion times bigger than the Solar System. At this time the Universe was almost smooth and expanding symmetrically in all directions. However, astronomers think that small clumps in density caused the formation of the first stars and galaxies. The first few seconds were so hot that the four basic forces (Gravity, Electromagnetic, Strong Nuclear, and Weak Nuclear) were unified. Gravity was the first force to separate itself from the other forces. Next, the weak nuclear force split from the others, allowing the formation of the first quarks, the building blocks of subatomic particles. Later on, the strong force split off and the first protons and neutrons were formed. After 100 seconds, it was cool enough for the first protons and neutrons started to link, however, the first atoms were very unstable and quickly broke apart. After three minutes the temperature dropped to a billion degrees and hydrogen and helium atoms formed. At the end of the Radiation era, the temperature dropped to 10,000K and the Universe became transparent in the Electromagnetic spectrum. A billion years later, the first stars and protogalaxies were formed.
Most people when they envision the Big Bang often think of it in an incorrect manner. They often envisioned looking at a small point of light that instantaneously becomes a tennis ball size of bright light. The next moment it becomes the size of a large geodesic dome of bright light, and from there they imagined it slowly expands to become the size of our universe. This, however, is not a good way of thinking about it. The reason for this is that you are imagining yourself outside of the small point of light. But as there is no outside so you cannot think of yourself as outside, you must think as if you were inside the point of light.
A better way to think of this is to imagine yourself in a very small and very dark cramped coffin (the singularity). All of the sudden the coffin becomes bright that is the size of the Earth (the tennis ball analogy). This happened so fast that you could not possibly know that it was happening. An instant later, so fast that you didn't know it happened, it becomes the size of the Sun (the geodesic dome analogy), and then from there it slowly expanded to the size of the today's Universe.
But this way of envisioning is not quite correct because you always envision yourself at the center of this expansion. We must also add another way of thinking about the expansion. Think of an uncooked loaf of bread which has raisins randomly spread throughout it. After putting the loaf of bread in an oven and heating it up the bread starts to expand, and the raisins move away from each other as part of the expansion of the loaf of bread. Now imagine yourself standing on one of those raisins inside the loaf of bread. If you looked at the other raisins, no matter in which direction you looked, it would appear that the other raisins are moving away from you, and you are the center of this movement away. However, if you had a friend standing on another raisin nearby and shouted to ask him why he was moving away from you, he would look at all the other raisins and it would seem to him that the other raisins were moving away from him. He would shout back that he is not moving, and that you are moving away from him. You would then get into an argument about who is moving away from whom. The answer to this argument is that the raisins are not moving, but the space between them is increasing. This provides the illusion that everything is expanding around an individual raisin, and that each raisin thinks the other raisin is moving away from them. By combining the coffin envisioning with the raisin envisioning you get a better impression of what the Big Bang looked like.
The Problems with the Standard Model!
While the Standard Model is a triumph of quantum physics and has withstood all observation and experimental tests, there are three major problems with the Standard Model as an explanation of how the Universe works.
The first is the problem of Gravity. Gravity is a universal force, but the "Standard Model" has no explanation for gravity, and gravity cannot incorporate the "Standard Model". Until Gravity and the "Standard Model" can be incorporated (through a "Grand Unified Theory" - GUT) it is not possible to have a full understanding of how and why the Universe works.
The second is the problem of Dark Matter. In the early 1990's Astrophysicists verified the existence of Dark Matter, matter that exists in the universe, but we cannot see. This was done by utilizing space telescopes to take a census of the stars and their star type in a galaxy to determine the approximate mass of the galaxy, then measuring the motion of selected stars through the galaxy, then feeding this information into a supercomputer that utilized Einstein's General Relativity equations to produce a gravitational model of the galaxy. To their surprise, the model said that the Galaxy could not exist because there was insufficient mass to hold it together. They did this for several galaxies, then dozens of galaxies, and every time the computer model said that the Galaxy could not exist. They adjusted the amount of mass in a galaxy in such a manner as to get the result that agreed with what they were observing in galaxies. In every case the adjustment was the same - the amount of normal matter (baryonic matter) was 20% of what was needed while 80% of the matter was unseen - which they named "Dark Matter". The astrophysicists went to the quantum physicists to ask what this Dark Matter could be. The quantum physicists had no answer. Yet everyone agrees that Dark Matter exists, and until the "Standard Model" can incorporate Dark Matter it will be incomplete.
The third is the problem of Dark Energy. In the late 1990's Astrophysicists realized it would be possible to measure the rate of expansion of the universe utilizing space telescopes and supercomputers (again utilizing Einstein's General Relativity equations). At that time they had three scenarios as to the ultimate fate of the universe; a closed universe, an open universe, or a flat universe. A closed universe is one in which the mass of the universe was greater than the force of expansion, and the universe would collapse onto itself to create a new universe (the expansion of space, a stop, and then the contraction of space). An open universe is one that the expansion is greater than the mass and the universe will expand forever and eventually suffer total radioactive decay and cease to exist. A flat universe is one in which the mass and the expansion are equal, and the universe would just stop and be fixed in size (nobody expected this result, but it was possible mathematically). Everybody expected that the rate of expansion was slowing, and we would end up in either an open or closed universe. To their surprise, the results showed that the rate of expansion was increasing. The only way this would be possible if there were a repulsive energy force that was greater than the gravitational force. They named this energy "Dark Energy". The astrophysicists went to the quantum physicists to ask what this Dark Energy could be. The quantum physicists had no answer. Yet everyone agrees that Dark Energy exists, and until the "Standard Model" can incorporate Dark Energy it will be incomplete.
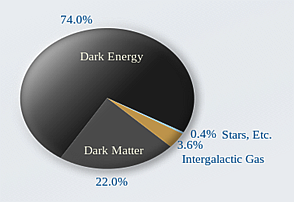 |
Given what we now know we can confidently say that about 74% of the Universe is composed of Dark Energy, and about 22% of the Universe is composed of Dark Matter, while only 4% of the Universe is Baryonic matter. This means that the "Standard Model of Quantum Physics" only accounts for 4% of what the universe is composed of. This situation, along with the unification of General Relativity and Quantum Physics, needs to be rectified to have a fuller understanding of how and why the Universe works. |
Final Thoughts
The modern technological world is not possible without Quantum Physics. All modern electronics must take into account Quantum Physics to function properly. The ubiquitous cell phone would not work without accounting for Quantum Physics (and General Relativity for GPS). Indeed, today's world would not be recognizable without Quantum Physics. We all owe a debt of gratitude for all those thousands of Quantum Physicists who were involved in the discovery and development of Quantum Physics. And finally, a touch of humor about Quantum Physics:
- I have difficulties trusting quantum physicist. Whenever two of them argues, they both lie and tell the truth at the same time.
- I was going to tell a joke about quantum mechanics but unfortunately by its very nature it's both funny and unfunny at the same time.
- So in another universe do I have a girlfriend?
- I am alive and dead at same time?
- When my mom walked in I immediately switched to porn cause it was easier to explain.
Further Readings
Below are the books I would recommend that you read for more background information on these scientists. They were chosen as they are a fairly easy read and have a minimum of mathematics. I would note that the first Book "The Clockwork Universe" should be read by even those not too interested in science, as it provides an excellent and fascinating look and the life, beliefs, politics, religion, and the state of knowledge of 17th Century England, as well as a narrative of the seventeenth century's scientific revolution.
- The Clockwork Universe: Isaac Newton, the Royal Society, and the Birth of the Modern World by Edward Dolnick
- Galileo: Decisive Innovator by Michael Sharratt
- The Life of Isaac Newton by Richard S. Westfall
- Faraday, Maxwell, and the Electromagnetic Field by Nancy Forbes & Basil Mahon
- Boltzmann's Atom by David Lindley
- Einstein - His Life and Universe by Walter Isaacson
- The Quantum Story - A History in 40 Moments by Jim Baggott
- The Atomic Scientists - A Biographical History by Henty A. Boorse, Lloyd Motz, & Jefferson Hane Weaver
- The Scientific 100 - A Ranking of the Most Influential Scientists, Past and Present by John Simmons
- Remarkable Mathematicians from Euler to von Neumann by Ioan James
- Physics for the Rest of Us - Ten Basic Ideas of 20th Century Physics by Roger S. Jones
For a brief introduction on these topics I would recommend the Oxford University Press series "A Very Short Introduction" on these subjects:
- Particle Physics: A Very Short Introduction by Frank Close
- Quantum Theory: A Very Short Introduction by John Polkinghorne
For some videos on these topics I would recommend:
- Isaac Newton's World of Physics
- The Extraordinary Genius of Albert Einstein
- The Secrets Of Quantum Physics
Some interesting website with general scientific topics are:
- Ask a Mathematician / Ask a Physicist
- Ask the Physicist!
- Physics and Astronomy Ask the Experts
- The Skeptic's Dictionary
- Understanding Science - how science really works
These timelines are from the website The Particle Adventure
Particle Physics Timeline
For over two thousand years people have thought about the fundamental particles from which all matter is made, starting with the gradual development of atomic theory, followed by a deeper understanding of the quantized atom, leading to the recent theory of the standard model.
We invite you to explore this history of particle physics with a focus on the scientists and thinkers who helped shape the field of particle physics. The four sections are arranged chronologically. You can use the index to find more information about a specific person or event.
Early Atomic Understanding
Earliest times - 1550 AD. The Greeks gave much to the world of physics by developing the basis of fundamental modern principles as the conservation of matter, atomic theory, and the like. Very few new developments occurred in the centuries following the Greek period. However, as the intense intellectual force of the Renaissance entered the field of physics, Copernicus and other great thinkers began to reject the Greek ideas in favor of new ideas based on empirical methods. Since Copernicus' theories ended the old era of scientific understanding as much as began the new scientific revolution, it is fitting to include him with the ancient thinkers.
|
624-547 B.C. |
Thales of Miletus postulates that water is the basic substance of the Earth. He also was acquainted with the attractive power of magnets and rubbed amber. |
|
580-500 B.C. |
Pythagoras held that the Earth was spherical. He sought a mathematical understanding of the universe. |
|
500-428 B.C., 484-424 B.C. |
Anaxagoras and Empedocles. Anaxagoras challenged the previous Greek contention about the creation and destruction of matter by teaching that changes in matter are due to different orderings of indivisible particles (thus his teachings were a precursor to the law of the conservation of matter). Empedocles reduced these indivisible particles into four elements: earth, air, fire, and water. |
|
460-370 B.C. |
Democritus developed the theory that the universe consists of empty space and an (almost) infinite number of invisible particles which differ from each other in form, position, and arrangement. All matter is made of indivisible particles called atoms. |
|
384-322 B.C. |
Aristotle formalized the gathering of scientific knowledge. While it is difficult to point to one particular theory, the total result of his compilation of knowledge was to provide the fundamental basis of science for a thousand years. |
|
310-230 B.C. |
Aristarchus describes a cosmology identical to that proposed by Copernicus 2,000 years later. However, given the great prestige of Aristotle, Aristarchus' heliocentric model was rejected in favor of the geocentric model. |
|
287-212 B.C. |
Archimedes was a great pioneer in theoretical physics. He provided the foundations of hydrostatics. |
|
70-147 AD |
Ptolemy of Alexandria collected the optical knowledge of the time. He also invented a complex theory of planetary motion. |
|
~1000 AD |
Alhazen, an Arab, produced 7 books on optics. |
|
1214-1294 AD |
Roger Bacon taught that in order to learn the secrets of nature we must first observe. He thus provided the method by which people can develop deductive theories using evidence from the natural world. |
|
1473-1543 AD |
Nicholaus Copernicus set forth the theory that the earth revolves around the sun. This heliocentric model was revolutionary in that it challenged the previous dogma of scientific authority of Aristotle, and caused a complete scientific and philosophical upheaval. |
The Scientific Revolution and Classical Mechanics Timeline
Following the Copernican revolution, it was apparent that scientific theories could not be accepted without rigorous testing. Communication among scientists increased and spurred more discoveries.
|
1564 - 1642 |
Galileo Galilei is considered by many to be the father of modern physics because of his willingness to replace old assumptions in favor of new scientifically deduced theories. He is famous for his celestial theories, and his works on mechanics paved the way for Newton. |
|
|
1546 - 1601, 1571 - 1630 |
Tycho Brahe and Johannes Kepler. Brahe's accurate celestial data allow Kepler to develop his theory of elliptical planetary motion and provide evidence for the Copernican system. In addition, Kepler writes a qualitative description of gravitation. |
|
|
1642 - 1727 |
Sir Isaac Newton develops the laws of mechanics (now called classical mechanics) which explains object motion in a mathematical fashion. |
|
|
1773 - 1829 |
Thomas Young develops the wave theory of light and describes light interference. |
|
|
1791 - 1867 |
Michael Faraday creates the electric motor and develops an understanding of electromagnetic induction, which provides evidence that electricity and magnetism are related. In addition, he discovers electrolysis and describes the conservation of energy law. |
|
|
1799 - 1878 |
Joesph Henry's research on electromagnetic induction is performed at the same time as Faraday's. He constructs the first motor; his work with electromagnets leads directly to the development of the telegraph. |
|
|
1873 |
James Clerk Maxwell performs important research in three areas: color vision, molecular theory, and electromagnetic theory. The ideas underlying Maxwell's theories of electromagnetism describes the propagation of light waves in a vacuum. |
|
|
1874 |
George Stoney develops a theory of the electron and estimates its mass. |
|
|
1895 |
Wilhelm Rontgen discovers x rays. |
|
|
1898 |
Marie and Pierre Curie separate radioactive elements. |
|
|
1898 |
Joseph Thompson measures the electron and puts forth his "plum-pudding" model of the atom -- that the atom is a slightly positive sphere with small, raisin-like negative electrons inside. |
Quantum Theory Timeline
At the start of the twentieth century, scientists believed that they understood the most fundamental principles of nature. Atoms were solid building blocks of nature; people trusted Newtonian laws of motion; most of the problems of physics seemed to be solved. However, starting with Einstein's theory of relativity which replaced Newtonian mechanics, scientists gradually realized that their knowledge was far from complete. Of particular interest was the growing field of quantum mechanics, which completely altered the fundamental precepts of physics.
Particles Discovered 1898 - 1964:
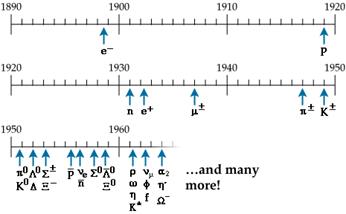
|
1900 |
Max Planck suggests that radiation is quantized (it comes in discrete amounts.) |
|
1905 |
Albert Einstein, one of the few scientists to take Planck's ideas seriously, proposes a quantum of light (the photon) which behaves like a particle. Einstein's other theories explained the equivalence of mass and energy, the particle-wave duality of photons, the equivalence principle, and special relativity. |
|
1909 |
Hans Geiger and Ernest Marsden, under the supervision of Ernest Rutherford, scatter alpha particles off a gold foil and observe large angles of scattering, suggesting that atoms have a small, dense, positively charged nucleus. |
|
1911 |
Ernest Rutherford infers the nucleus as the result of the alpha-scattering experiment performed by Hans Geiger and Ernest Marsden. |
|
1912 |
Albert Einstein explains the curvature of space-time. |
|
1913 |
Niels Bohr succeeds in constructing a theory of atomic structure based on quantum ideas. |
|
1919 |
Ernest Rutherford finds the first evidence for a proton. |
|
1921 |
James Chadwick and E.S. Bieler conclude that some strong force holds the nucleus together. |
|
1923 |
Arthur Compton discovers the quantum (particle) nature of x rays, thus confirming photons as particles. |
|
1924 |
Louis de Broglie proposes that matter has wave properties. |
|
1925 (Jan) |
Wolfgang Pauli formulates the exclusion principle for electrons in an atom. |
|
1925 (April) |
Walther Bothe and Hans Geiger demonstrate that energy and mass are conserved in atomic processes. |
|
1926 |
Erwin Schroedinger develops wave mechanics, which describes the behavior of quantum systems for bosons. Max Born gives a probability interpretation of quantum mechanics. G.N. Lewis proposes the name "photon" for a light quantum. |
|
1927 |
Certain materials had been observed to emit electrons (beta decay). Since both the atom and the nucleus have discrete energy levels, it is hard to see how electrons produced in transition could have a continuous spectrum (see 1930 for an answer.) |
|
1927 |
Werner Heisenberg formulates the uncertainty principle: the more you know about a particle's energy, the less you know about the time of the energy (and vice versa.) The same uncertainty applies to momenta and coordinates. |
|
1928 |
Paul Dirac combines quantum mechanics and special relativity to describe the electron. |
|
1930 |
Quantum mechanics and special relativity are well established. There are just three fundamental particles: protons, electrons, and photons. Max Born, after learning of the Dirac equation, said, "Physics as we know it will be over in six months." |
|
1930 |
Wolfgang Pauli suggests the neutrino to explain the continuous electron spectrum for beta decay. |
|
1931 |
Paul Dirac realizes that the positively-charged particles required by his equation are new objects (he calls them "positrons"). They are exactly like electrons, but positively charged. This is the first example of antiparticles. |
|
1931 |
James Chadwick discovers the neutron. The mechanisms of nuclear binding and decay become primary problems. |
|
1933-34 |
Enrico Fermi puts forth a theory of beta decay that introduces the weak interaction. This is the first theory to explicitly use neutrinos and particle flavor changes. |
|
1933-34 |
Hideki Yukawa combines relativity and quantum theory to describe nuclear interactions by an exchange of new particles (mesons called "pions") between protons and neutrons. From the size of the nucleus, Yukawa concludes that the mass of the conjectured particles (mesons) is about 200 electron masses. This is the beginning of the meson theory of nuclear forces. |
|
1937 |
A particle of 200 electron masses is discovered in cosmic rays. While at first physicists thought it was Yukawa's pion, it was later discovered to be a muon. |
|
1938 |
E.C.G. St??ckelberg observes that protons and neutrons do not decay into any combination of electrons, neutrinos, muons, or their antiparticles. The stability of the proton cannot be explained in terms of energy or charge conservation; he proposes that heavy particles are independently conserved. |
|
1941 |
C. Moller and Abraham Pais introduce the term "nucleon" as a generic term for protons and neutrons. |
|
1946-47 |
Physicists realize that the cosmic ray particle thought to be Yukawa's meson is instead a "muon," the first particle of the second generation of matter particles to be found. This discovery was completely unexpected -- I.I. Rabicomments "who ordered that?" The term "lepton" is introduced to describe objects that do not interact too strongly (electrons and muons are both leptons). |
|
1947 |
A meson that does interact strongly is found in cosmic rays, and is determined to be the pion. |
|
1947 |
Physicists develop procedures to calculate electromagnetic properties of electrons, positrons, and photons. Introduction of Feynman diagrams. |
|
1948 |
The Berkeley synchro-cyclotron produces the first artificial pions. |
|
1949 |
Enrico Fermi and C.N. Yang suggest that a pion is a composite structure of a nucleon and an anti-nucleon. This idea of composite particles is quite radical. |
|
1949 |
Discovery of K+ via its decay. |
|
1950 |
The neutral pion is discovered. |
|
1951 |
Two new types of particles are discovered in cosmic rays. They are discovered by looking at V-like tracks and reconstructing the electrically-neutral object that must have decayed to produce the two charged objects that left the tracks. The particles were named the lambda0 and the K0. |
|
1952 |
Discovery of particle called delta: there were four similar particles (delta++, delta+, delta0, and delta-.) |
|
1952 |
Donald Glaser invents the bubble chamber. The Brookhaven Cosmotron, a 1.3 GeV accelerator, starts operation. |
|
1953 |
The beginning of a "particle explosion" -- a true proliferation of particles. |
|
1953 - 57 |
Scattering of electrons off nuclei reveals a charge density distribution inside protons and even neutrons. Description of this electromagnetic structure of protons and neutrons suggests some kind of internal structure to these objects, though they are still regarded as fundamental particles. |
|
1954 |
C.N. Yang and Robert Mills develop a new class of theories called "gauge theories." Although not realized at the time, this type of theory now forms the basis of the Standard Model. |
|
1957 |
Julian Schwinger writes a paper proposing a unification of weak and electromagnetic interactions. |
|
1957-59 |
Julian Schwinger, Sidney Bludman, and Sheldon Glashow, in separate papers, suggest that all weak interactions are mediated by charged heavy bosons, later called W+ and W-. Actually, it was Yukawa who first discussed boson exchange twenty years earlier, but he proposed the pion as the mediator of the weak force. |
|
1961 |
As the number of known particles increased, a mathematical classification scheme to organize the particles (the group SU(3)) helps physicists recognize patterns of particle types. |
|
1962 |
Experiments verify that there are two distinct types of neutrinos (electron and muon neutrinos). This was earlier inferred from theoretical considerations. |
Modern View (Standard Model) Timeline
By the mid-1960's, physicists realized that their previous understanding, where all matter is composed of the fundamental protons, neutrons, and electron, was insufficient to explain the myriad new particles being discovered. Gell-Mann's and Zweig's quark theory solved these problems. Over the last thirty years, the theory that is now called the standard model of particles and interactions has gradually grown and gained increasing acceptance with new evidence from new particle accelerators.
Particles discovered 1964 - present:
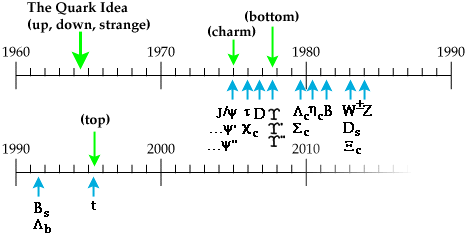
|
1964 |
Murray Gell-Mann and George Zweig tentatively put forth the idea of quarks. They suggested that mesons and baryons are composites of three quarks or antiquarks, called up, down, or strange (u, d, s) with spin 0.5 and electric charges 2/3, -1/3, -1/3, respectively (it turns out that this theory is not completely accurate). Since the charges had never been observed, the introduction of quarks was treated more as a mathematical explanation of flavor patterns of particle masses than as a postulate of actual physical object. Later theoretical and experimental developments allow us to now regard the quarks as real physical objects, even though they cannot be isolated. |
|
1964 |
Since leptons had a certain pattern, several papers suggested a fourth quark carrying another flavor to give a similar repeated pattern for the quarks, now seen as the generations of matter. Very few physicists took this suggestion seriously at the time. Sheldon Glashow and James Bjorken coin the term "charm" for the fourth (c) quark. |
|
1965 |
O.W. Greenberg, M.Y. Han, and Yoichiro Nambu introduce the quark property of color charge. All observed hadrons are color neutral. |
|
...1966... |
The quark model is accepted rather slowly because quarks hadn't been observed. |
|
1967 |
Steven Weinberg and Abdus Salam separately propose a theory that unifies electromagnetic and weak interactions into the electroweak interaction. Their theory requires the existence of a neutral, weakly interacting boson (now called the Z0) that mediates a weak interaction that had not been observed at that time. They also predict an additional massive boson called the Higgs Boson that has not yet been observed. |
|
1968-69 |
At the Stanford Linear Accelerator, in an experiment in which electrons are scattered off protons, the electrons appear to be bouncing off small hard cores inside the proton. James Bjorken and Richard Feynman analyze this data in terms of a model of constituent particles inside the proton (they didn't use the name "quark" for the constituents, even though this experiment provided evidence for quarks.) |
|
1970 |
Sheldon Glashow, John Iliopoulos, and Luciano Maiani recognize the critical importance of a fourth type of quark in the context of the Standard Model. A fourth quark allows a theory that has flavor-conserving Z0-mediated weak interactions but no flavor-changing ones. |
|
1973 |
Donald Perkins, spurred by a prediction of the Standard Model, re-analyzes some old data from CERN and finds indications of weak interactions with no charge exchange (those due to a Z0 exchange.) |
|
1973 |
A quantum field theory of strong interaction is formulated. This theory of quarks and gluons (now part of the Standard Model) is similar in structure to quantum electrodynamics (QED), but since strong interaction deals with color charge this theory is called quantum chromodynamics (QCD). Quarks are determined to be real particles, carrying a color charge. Gluons are massless quanta of the strong-interaction field. This strong interaction theory was first suggested by Harald Fritzsch and Murray Gell-Mann. |
|
1973 |
David Politzer, David Gross, and Frank Wilczek discover that the color theory of the strong interaction has a special property, now called "asymptotic freedom." The property is necessary to describe the 1968-69 data on the substrate of the proton. |
|
1974 |
In a summary talk for a conference, John Iliopoulos presents, for the first time in a single report, the view of physics now called the Standard Model. If you want to understand the various aspects of the Standard Model, please explore the Standard Model Path. |
|
1974 (Nov.) |
Burton Richter and Samuel Ting, leading independent experiments, announce on the same day that they discovered the same new particle. Ting and his collaborators at Brookhaven called this particle the "J" particle, whereas Richter and his collaborators at SLAC called this particle the psi particle. Since the discoveries are given equal weight, the particle is commonly known as the J/psi particle. The J/psi particle is a charm-anticharm meson. |
|
1976 |
Gerson Goldhaber and Francois Pierre find the D0 meson (anti-up and charm quarks). The theoretical predictions agreed dramatically with the experimental results, offering support for the Standard Model. |
|
1976 |
The tau lepton is discovered by Martin Perl and collaborators at SLAC. Since this lepton is the first recorded particle of the third generation, it is completely unexpected. |
|
1977 |
Leon Lederman and his collaborators at Fermilab discover yet another quark (and its antiquark). This quark was called the "bottom" quark. Since physicists figured that quarks came in pairs, this discovery adds impetus to search for the sixth quark -- "top." |
|
1978 |
Charles Prescott and Richard Taylor observe a Z0 mediated weak interaction in the scattering of polarized electrons from deuterium which shows a violation of parity conservation, as predicted by the Standard Model, confirming the theory's prediction. |
|
1979 |
Strong evidence for a gluon radiated by the initial quark or antiquark if found at PETRA, a colliding beam facility at the DESY laboratory in Hamburg, |
|
1983 |
The W?? and Z0 intermediate bosons demanded by the electroweak theory are observed by two experiments using the CERN synchrotron using techniques developed by Carlo Rubbia and Simon Van der Meer to collide protons and antiprotons. |
|
1989 |
Experiments carried out in SLAC and CERN strongly suggest that there are three and only three generations of fundamental particles. This is inferred by showing that the Z0-boson lifetime is consistent only with the existence of exactly three very light (or massless) neutrinos. |
|
1995 |
After eighteen years of searching at many accelerators, the CDF and D0 experiments at Fermilab discover the top quark at the unexpected mass of 175 GeV. No one understands why the mass is so different from the other five quarks. |
|
2012 |
Almost half a century after Peter Higgs predicted a Higgs boson as part of a mechanism (invented by a number of theorists) by which fundamental particles gain mass, the ATLAS and CMS experiments at the CERN lab discover the Higgs boson. |
The Noble Prize in Physics
Practically of of the Noble prize winners in physics had an impact on Atomic Physics. For this reason, I have included a list of these winners. The Nobel Prize in Physics has been awarded 111 times to 207 Nobel Laureates between 1901 and 2017. John Bardeen is the only Nobel Laureate who has been awarded the Nobel Prize in Physics twice, in 1956 and 1972. This means that a total of 206 individuals have received the Nobel Prize in Physics.
Please visit the Nobelprize.org the Official Web Site of the Nobel Prize for more information as to their contribution to Atomic Physics and their being awarded the Noble Prize.
2022 - Alain Aspect, John F. Clauser and Anton Zeilinger ?for experiments with entangled photons, establishing the violation of Bell inequalities and pioneering quantum information science?
2021 - ?for groundbreaking contributions to our understanding of complex systems?
Syukuro Manabe and Klaus Hasselmann ?for the physical modelling of Earth?s climate, quantifying variability and reliably predicting global warming?
Giorgio Parisi ?for the discovery of the interplay of disorder and fluctuations in physical systems from atomic to planetary scales?
2020 - Roger Penrose ?for the discovery that black hole formation is a robust prediction of the general theory of relativity?
Reinhard Genzel and Andrea Ghez ?for the discovery of a supermassive compact object at the centre of our galaxy?
2019 - ?for contributions to our understanding of the evolution of the universe and Earth?s place in the cosmos?
James Peebles ?for theoretical discoveries in physical cosmology?
Michel Mayor and Didier Queloz ?for the discovery of an exoplanet orbiting a solar-type star?
2018 - ?for groundbreaking inventions in the field of laser physics?
Arthur Ashkin ?for the optical tweezers and their application to biological systems?
G?rard Mourou and Donna Strickland ?for their method of generating high-intensity, ultra-short optical pulses?
2017 - Rainer Weiss, Barry C. Barish and Kip S. Thorne ?for decisive contributions to the LIGO detector and the observation of gravitational waves?
2016 - David J. Thouless, F. Duncan M. Haldane and J. Michael Kosterlitz ?for theoretical discoveries of topological phase transitions and topological phases of matter?
2015 - Takaaki Kajita and Arthur B. McDonald ?for the discovery of neutrino oscillations, which shows that neutrinos have mass?
2014 - Isamu Akasaki, Hiroshi Amano and Shuji Nakamura ?for the invention of efficient blue light-emitting diodes which has enabled bright and energy-saving white light sources?
2013 - Fran?ois Englert and Peter W. Higgs ?for the theoretical discovery of a mechanism that contributes to our understanding of the origin of mass of subatomic particles, and which recently was confirmed through the discovery of the predicted fundamental particle, by the ATLAS and CMS experiments at CERN?s Large Hadron Collider?
2012 - Serge Haroche and David J. Wineland ?for ground-breaking experimental methods that enable measuring and manipulation of individual quantum systems?
2011 - Saul Perlmutter, Brian P. Schmidt and Adam G. Riess ?for the discovery of the accelerating expansion of the Universe through observations of distant supernovae?
2010 - Andre Geim and Konstantin Novoselov ?for groundbreaking experiments regarding the two-dimensional material graphene?
2009 - Charles Kuen Kao ?for groundbreaking achievements concerning the transmission of light in fibers for optical communication?
Willard S. Boyle and George E. Smith ?for the invention of an imaging semiconductor circuit ? the CCD sensor?
2008 - Yoichiro Nambu ?for the discovery of the mechanism of spontaneous broken symmetry in subatomic physics?
Makoto Kobayashi and Toshihide Maskawa ?for the discovery of the origin of the broken symmetry which predicts the existence of at least three families of quarks in nature?
2007 - Albert Fert and Peter Gr?nberg ?for the discovery of Giant Magnetoresistance?
2006 - John C. Mather and George F. Smoot ?for their discovery of the blackbody form and anisotropy of the cosmic microwave background radiation?
2005 - Roy J. Glauber ?for his contribution to the quantum theory of optical coherence?
John L. Hall and Theodor W. H?nsch ?for their contributions to the development of laser-based precision spectroscopy, including the optical frequency comb technique?
2004 - David J. Gross, H. David Politzer and Frank Wilczek ?for the discovery of asymptotic freedom in the theory of the strong interaction?
2003 - Alexei A. Abrikosov, Vitaly L. Ginzburg and Anthony J. Leggett ?for pioneering contributions to the theory of superconductors and superfluids?
2002 - Raymond Davis Jr. and Masatoshi Koshiba ?for pioneering contributions to astrophysics, in particular for the detection of cosmic neutrinos?
Riccardo Giacconi ?for pioneering contributions to astrophysics, which have led to the discovery of cosmic X-ray sources?
2001 - Eric A. Cornell, Wolfgang Ketterle and Carl E. Wieman ?for the achievement of Bose-Einstein condensation in dilute gases of alkali atoms, and for early fundamental studies of the properties of the condensates?
2000 - ?for basic work on information and communication technology?
Zhores I. Alferov and Herbert Kroemer ?for developing semiconductor heterostructures used in high-speed- and opto-electronics?
Jack S. Kilby ?for his part in the invention of the integrated circuit?
1999 - Gerardus ?t Hooft and Martinus J.G. Veltman ?for elucidating the quantum structure of electroweak interactions in physics?
1998 - Robert B. Laughlin, Horst L. St?rmer and Daniel C. Tsui ?for their discovery of a new form of quantum fluid with fractionally charged excitations?
1997 - Steven Chu, Claude Cohen-Tannoudji and William D. Phillips ?for development of methods to cool and trap atoms with laser light?
1996 - David M. Lee, Douglas D. Osheroff and Robert C. Richardson ?for their discovery of superfluidity in helium-3?
1995 - ?for pioneering experimental contributions to lepton physics?
Martin L. Perl ?for the discovery of the tau lepton?
Frederick Reines ?for the detection of the neutrino?
1994 - ?for pioneering contributions to the development of neutron scattering techniques for studies of condensed matter?
Bertram N. Brockhouse ?for the development of neutron spectroscopy?
Clifford G. Shull ?for the development of the neutron diffraction technique?
1993 - Russell A. Hulse and Joseph H. Taylor Jr. ?for the discovery of a new type of pulsar, a discovery that has opened up new possibilities for the study of gravitation?
1992 - Georges Charpak ?for his invention and development of particle detectors, in particular the multiwire proportional chamber?
1991 - Pierre-Gilles de Gennes ?for discovering that methods developed for studying order phenomena in simple systems can be generalized to more complex forms of matter, in particular to liquid crystals and polymers?
1990 - Jerome I. Friedman, Henry W. Kendall and Richard E. Taylor ?for their pioneering investigations concerning deep inelastic scattering of electrons on protons and bound neutrons, which have been of essential importance for the development of the quark model in particle physics?
1989 - Norman F. Ramsey ?for the invention of the separated oscillatory fields method and its use in the hydrogen maser and other atomic clocks?
Hans G. Dehmelt and Wolfgang Paul ?for the development of the ion trap technique?
1988 - Leon M. Lederman, Melvin Schwartz and Jack Steinberger ?for the neutrino beam method and the demonstration of the doublet structure of the leptons through the discovery of the muon neutrino?
1987 - J. Georg Bednorz and K. Alexander M?ller ?for their important break-through in the discovery of superconductivity in ceramic materials?
1986 - Ernst Ruska ?for his fundamental work in electron optics, and for the design of the first electron microscope?
Gerd Binnig and Heinrich Rohrer ?for their design of the scanning tunneling microscope?
1985 - Klaus von Klitzing ?for the discovery of the quantized Hall effect?
1984 - Carlo Rubbia and Simon van der Meer ?for their decisive contributions to the large project, which led to the discovery of the field particles W and Z, communicators of weak interaction?
1983 - Subramanyan Chandrasekhar ?for his theoretical studies of the physical processes of importance to the structure and evolution of the stars?
William Alfred Fowler ?for his theoretical and experimental studies of the nuclear reactions of importance in the formation of the chemical elements in the universe?
1982 - Kenneth G. Wilson ?for his theory for critical phenomena in connection with phase transitions?
1981 - Nicolaas Bloembergen and Arthur Leonard Schawlow ?for their contribution to the development of laser spectroscopy?
Kai M. Siegbahn ?for his contribution to the development of high-resolution electron spectroscopy?
1980 - James Watson Cronin and Val Logsdon Fitch ?for the discovery of violations of fundamental symmetry principles in the decay of neutral K-mesons?
1979 - Sheldon Lee Glashow, Abdus Salam and Steven Weinberg ?for their contributions to the theory of the unified weak and electromagnetic interaction between elementary particles, including, inter alia, the prediction of the weak neutral current?
1978 - Pyotr Leonidovich Kapitsa ?for his basic inventions and discoveries in the area of low-temperature physics?
Arno Allan Penzias and Robert Woodrow Wilson ?for their discovery of cosmic microwave background radiation?
1977 - Philip Warren Anderson, Sir Nevill Francis Mott and John Hasbrouck Van Vleck ?for their fundamental theoretical investigations of the electronic structure of magnetic and disordered systems?
1976 - Burton Richter and Samuel Chao Chung Ting ?for their pioneering work in the discovery of a heavy elementary particle of a new kind?
1975 - Aage Niels Bohr, Ben Roy Mottelson and Leo James Rainwater ?for the discovery of the connection between collective motion and particle motion in atomic nuclei and the development of the theory of the structure of the atomic nucleus based on this connection?
1974 - Sir Martin Ryle and Antony Hewish ?for their pioneering research in radio astrophysics: Ryle for his observations and inventions, in particular of the aperture synthesis technique, and Hewish for his decisive role in the discovery of pulsars?
1973 - Leo Esaki and Ivar Giaever ?for their experimental discoveries regarding tunneling phenomena in semiconductors and superconductors, respectively?
Brian David Josephson ?for his theoretical predictions of the properties of a supercurrent through a tunnel barrier, in particular those phenomena which are generally known as the Josephson effects?
1972 - John Bardeen, Leon Neil Cooper and John Robert Schrieffer ?for their jointly developed theory of superconductivity, usually called the BCS-theory?
1971 - Dennis Gabor ?for his invention and development of the holographic method?
1970 - Hannes Olof G?sta Alfv?n ?for fundamental work and discoveries in magnetohydro-dynamics with fruitful applications in different parts of plasma physics?
Louis Eug?ne F?lix N?el ?for fundamental work and discoveries concerning antiferromagnetism and ferrimagnetism which have led to important applications in solid state physics?
1969 - Murray Gell-Mann ?for his contributions and discoveries concerning the classification of elementary particles and their interactions?
1968 - Luis Walter Alvarez ?for his decisive contributions to elementary particle physics, in particular the discovery of a large number of resonance states, made possible through his development of the technique of using hydrogen bubble chamber and data analysis?
1967 - Hans Albrecht Bethe ?for his contributions to the theory of nuclear reactions, especially his discoveries concerning the energy production in stars?
1966 - Alfred Kastler ?for the discovery and development of optical methods for studying Hertzian resonances in atoms?
1965 - Sin-Itiro Tomonaga, Julian Schwinger and Richard P. Feynman ?for their fundamental work in quantum electrodynamics, with deep-ploughing consequences for the physics of elementary particles?
1964 - Charles Hard Townes, Nicolay Gennadiyevich Basov and Aleksandr Mikhailovich Prokhorov ?for fundamental work in the field of quantum electronics, which has led to the construction of oscillators and amplifiers based on the maser-laser principle?
1963 - Eugene Paul Wigner ?for his contributions to the theory of the atomic nucleus and the elementary particles, particularly through the discovery and application of fundamental symmetry principles?
Maria Goeppert Mayer and J. Hans D. Jensen ?for their discoveries concerning nuclear shell structure?
1962 - Lev Davidovich Landau ?for his pioneering theories for condensed matter, especially liquid helium?
1961 - Robert Hofstadter ?for his pioneering studies of electron scattering in atomic nuclei and for his thereby achieved discoveries concerning the structure of the nucleons?
Rudolf Ludwig M?ssbauer ?for his researches concerning the resonance absorption of gamma radiation and his discovery in this connection of the effect which bears his name?
1960 - Donald Arthur Glaser ?for the invention of the bubble chamber?
1959 - Emilio Gino Segr? and Owen Chamberlain ?for their discovery of the antiproton?
1958 - Pavel Alekseyevich Cherenkov, Il?ja Mikhailovich Frank and Igor Yevgenyevich Tamm ?for the discovery and the interpretation of the Cherenkov effect?
1957 - Chen Ning Yang and Tsung-Dao (T.D.) Lee ?for their penetrating investigation of the so-called parity laws which has led to important discoveries regarding the elementary particles?
1956 - William Bradford Shockley, John Bardeen and Walter Houser Brattain ?for their researches on semiconductors and their discovery of the transistor effect?
1955 - Willis Eugene Lamb ?for his discoveries concerning the fine structure of the hydrogen spectrum?
Polykarp Kusch ?for his precision determination of the magnetic moment of the electron?
1954 - Max Born ?for his fundamental research in quantum mechanics, especially for his statistical interpretation of the wavefunction?
Walther Bothe ?for the coincidence method and his discoveries made therewith?
1953 - Frits Zernike ?for his demonstration of the phase contrast method, especially for his invention of the phase contrast microscope?
1952 - Felix Bloch and Edward Mills Purcell ?for their development of new methods for nuclear magnetic precision measurements and discoveries in connection therewith?
1951 - Sir John Douglas Cockcroft and Ernest Thomas Sinton Walton ?for their pioneer work on the transmutation of atomic nuclei by artificially accelerated atomic particles?
1950 - Cecil Frank Powell ?for his development of the photographic method of studying nuclear processes and his discoveries regarding mesons made with this method?
1949 - Hideki Yukawa ?for his prediction of the existence of mesons on the basis of theoretical work on nuclear forces?
1948 - Patrick Maynard Stuart Blackett ?for his development of the Wilson cloud chamber method, and his discoveries therewith in the fields of nuclear physics and cosmic radiation?
1947 - Sir Edward Victor Appleton ?for his investigations of the physics of the upper atmosphere especially for the discovery of the so-called Appleton layer?
1946 - Percy Williams Bridgman ?for the invention of an apparatus to produce extremely high pressures, and for the discoveries he made therewith in the field of high pressure physics?
1945 - Wolfgang Pauli ?for the discovery of the Exclusion Principle, also called the Pauli Principle?
1944 - Isidor Isaac Rabi ?for his resonance method for recording the magnetic properties of atomic nuclei?
1943 - Otto Stern ?for his contribution to the development of the molecular ray method and his discovery of the magnetic moment of the proton?
1942 - No Nobel Prize was awarded this year. The prize money was with 1/3 allocated to the Main Fund and with 2/3 to the Special Fund of this prize section.
1941 - No Nobel Prize was awarded this year. The prize money was with 1/3 allocated to the Main Fund and with 2/3 to the Special Fund of this prize section.
1940 - No Nobel Prize was awarded this year. The prize money was with 1/3 allocated to the Main Fund and with 2/3 to the Special Fund of this prize section.
1939 - Ernest Orlando Lawrence ?for the invention and development of the cyclotron and for results obtained with it, especially with regard to artificial radioactive elements?
1938 - Enrico Fermi ?for his demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons?
1937 - Clinton Joseph Davisson and George Paget Thomson ?for their experimental discovery of the diffraction of electrons by crystals?
1936 - Victor Franz Hess ?for his discovery of cosmic radiation?
Carl David Anderson ?for his discovery of the positron?
1935 - James Chadwick?for the discovery of the neutron?
1934 - No Nobel Prize was awarded this year. The prize money was with 1/3 allocated to the Main Fund and with 2/3 to the Special Fund of this prize section.
1933 - Erwin Schr?dinger and Paul Adrien Maurice Dirac ?for the discovery of new productive forms of atomic theory?
1932 - Werner Karl Heisenberg ?for the creation of quantum mechanics, the application of which has, inter alia, led to the discovery of the allotropic forms of hydrogen?
1931 - No Nobel Prize was awarded this year. The prize money was allocated to the Special Fund of this prize section.
1930 - Sir Chandrasekhara Venkata Raman ?for his work on the scattering of light and for the discovery of the effect named after him?
1929 - Prince Louis-Victor Pierre Raymond de Broglie ?for his discovery of the wave nature of electrons?
1928 - Owen Willans Richardson ?for his work on the thermionic phenomenon and especially for the discovery of the law named after him?
1927 - Arthur Holly Compton ?for his discovery of the effect named after him?
Charles Thomson Rees Wilson ?for his method of making the paths of electrically charged particles visible by condensation of vapour?
1926 - Jean Baptiste Perrin ?for his work on the discontinuous structure of matter, and especially for his discovery of sedimentation equilibrium?
1925 - James Franck and Gustav Ludwig Hertz ?for their discovery of the laws governing the impact of an electron upon an atom?
1924 - Karl Manne Georg Siegbahn ?for his discoveries and research in the field of X-ray spectroscopy?
1923 - Robert Andrews Millikan ?for his work on the elementary charge of electricity and on the photoelectric effect?
1922 - Niels Henrik David Bohr ?for his services in the investigation of the structure of atoms and of the radiation emanating from them?
1921 - Albert Einstein ?for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect?
1920 - Charles Edouard Guillaume ?in recognition of the service he has rendered to precision measurements in Physics by his discovery of anomalies in nickel steel alloys?
1919 - Johannes Stark ?for his discovery of the Doppler effect in canal rays and the splitting of spectral lines in electric fields?
1918 - Max Karl Ernst Ludwig Planck ?in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta?
1917 - Charles Glover Barkla ?for his discovery of the characteristic R?ntgen radiation of the elements?
1916 - No Nobel Prize was awarded this year. The prize money was allocated to the Special Fund of this prize section.
1915 - Sir William Henry Bragg and William Lawrence Bragg ?for their services in the analysis of crystal structure by means of X-rays?
1914 - Max von Laue ?for his discovery of the diffraction of X-rays by crystals?
1913 - Heike Kamerlingh Onnes ?for his investigations on the properties of matter at low temperatures which led, inter alia, to the production of liquid helium?
1912 - Nils Gustaf Dal?n ?for his invention of automatic regulators for use in conjunction with gas accumulators for illuminating lighthouses and buoys?
1911 - Wilhelm Wien ?for his discoveries regarding the laws governing the radiation of heat?
1910 - Johannes Diderik van der Waals ?for his work on the equation of state for gases and liquids?
1909 - Guglielmo Marconi and Karl Ferdinand Braun ?in recognition of their contributions to the development of wireless telegraphy?
1908 - Gabriel Lippmann ?for his method of reproducing colours photographically based on the phenomenon of interference?
1907 - Albert Abraham Michelson ?for his optical precision instruments and the spectroscopic and metrological investigations carried out with their aid?
1906 - Joseph John Thomson ?in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases?
1905 - Philipp Eduard Anton von Lenard ?for his work on cathode rays?
1904 - Lord Rayleigh (John William Strutt) ?for his investigations of the densities of the most important gases and for his discovery of argon in connection with these studies?
1903 - Antoine Henri Becquerel ?in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity?
Pierre Curie and Marie Curie, n?e Sklodowska ?in recognition of the extraordinary services they have rendered by their joint researches on the radiation phenomena discovered by Professor Henri Becquerel?
1902 - Hendrik Antoon Lorentz and Pieter Zeeman ?in recognition of the extraordinary service they rendered by their researches into the influence of magnetism upon radiation phenomena?
1901 - Wilhelm Conrad R?ntgen?in recognition of the extraordinary services he has rendered by the discovery of the remarkable rays subsequently named after him? -
Disclaimer
Please Note - many academics, scientist and engineers would critique what I have written here as not accurate nor through. I freely acknowledge that these critiques are correct. It was not my intentions to be accurate or through, as I am not qualified to give an accurate nor through description. My intention was to be understandable to a layperson so that they can grasp the concepts. Academics, scientists, and engineers entire education and training is based on accuracy and thoroughness, and as such, they strive for this accuracy and thoroughness. I believe it is essential for all laypersons to grasp the concepts of this paper, so they make more informed decisions on those areas of human endeavors that deal with this subject. As such, I did not strive for accuracy and thoroughness, only understandability.
Most academics, scientist, and engineers when speaking or writing for the general public (and many science writers as well) strive to be understandable to the general public. However, they often fall short on the understandability because of their commitment to accuracy and thoroughness, as well as some audience awareness factors. Their two biggest problems are accuracy and the audience knowledge of the topic.
Accuracy is a problem because academics, scientist, engineers and science writers are loath to be inaccurate. This is because they want the audience to obtain the correct information, and the possible negative repercussions amongst their colleagues and the scientific community at large if they are inaccurate. However, because modern science is complex this accuracy can, and often, leads to confusion amongst the audience.
The audience knowledge of the topic is important as most modern science is complex, with its own words, terminology, and basic concepts the audience is unfamiliar with, or they misinterpret. The audience becomes confused (even while smiling and lauding the academics, scientists, engineers or science writer), and the audience does not achieve understandability. Many times, the academics, scientists, engineers or science writer utilizes the scientific disciplines own words, terminology, and basic concepts without realizing the audience misinterpretations, or has no comprehension of these items.
It is for this reason that I place understandability as the highest priority in my writing, and I am willing to sacrifice accuracy and thoroughness to achieve understandability. There are many books, websites, and videos available that are more accurate and through. The subchapter on "Further Readings" also contains books on various subjects that can provide more accurate and thorough information. I leave it to the reader to decide if they want more accurate or through information and to seek out these books, websites, and videos for this information.
If you have any comments, concerns, critiques, or suggestions I can be reached at mwd@profitpages.com.
I will review reasoned and intellectual correspondence, and it is possible that I can change my mind,
or at least update the content of this article.